Chemical - Bonding-8
Predicting The Hybrid State of Central Atom
The hybrid state of central atoms in simple molecule or in plyatomic ion can be easily predicted by the following considerations :
(i) Count the number of atoms/groups surrounding the central atom. Let it be (SA)
(ii) Find the valence electrons of central atom from its atomic number. Let it be (G)
(iii) Find the valency of the central atom in the species. Let it be (V).
V is equal to the number of monovalent groups such as H, Cl, Br, OH ....., etc., or double the numbers of divalent groups such as O, S, ..... etc., directly bonded to central atom.
(iv) If the given species is anionic, the charge on the ion is represented by (a) whereas as if it is cation, the charge on it represented by c.
Now SA, G, V, E are related as :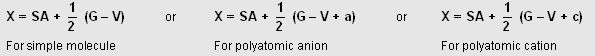
Now, hybrid state, shape, etc., can be prepicted from the following table :
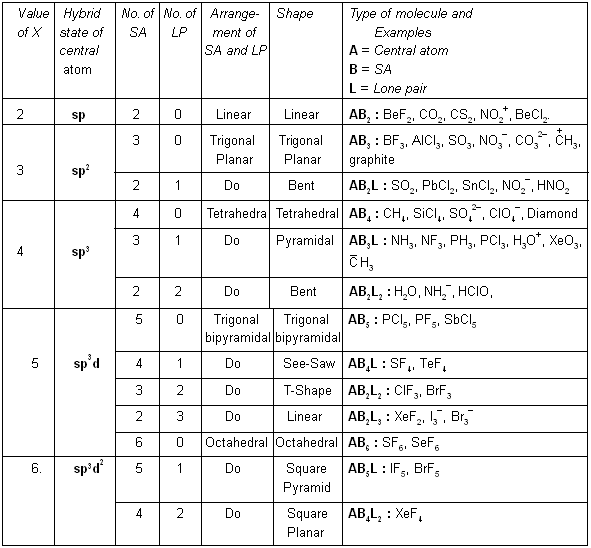
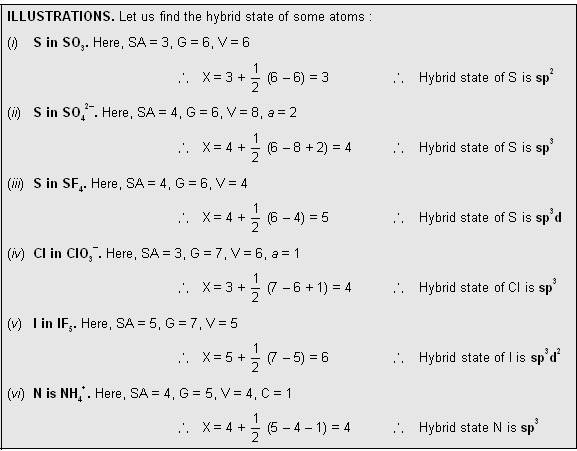
In 1940, Sidgwick and Powell suggested that the shape of a molecule is related to the number of electrons in the outer shell of the central atom. Irrespective of the fact whether bond pairs or lone pairs occupy the orbitals, the occupied orbitals repel each other and consequently are oriented in space as far apart as possible. In this position molecule has minimum energy, and therefore, maximum stability. The shape of the molecule and bond angles can be predicted if the distribution of orbitals about the central atom can be ascertained.