common-names-geneva-system-1
IUPAC (Geneva System of Nomenclature)
(i) Regular pattern of naming
| side chain + alk + (-)ane or (=)ene or (=)yne + suffix of main functional group | |
| 1C | 4C |
| 2C | 5C |
| 3C | 6C |
(ii) Selection of LPCC ( longest possible carbon chain)
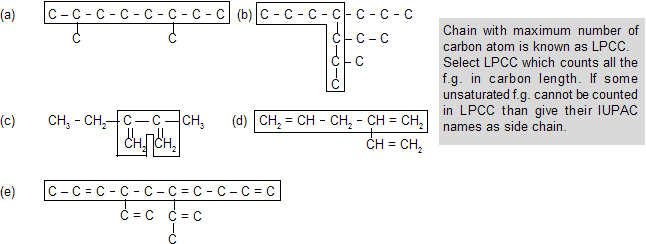
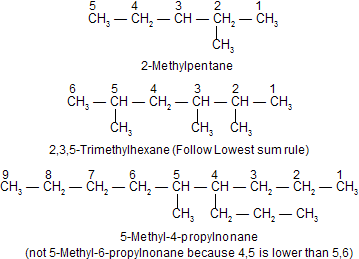
The longest possible chain is numbered from one side to the other by Arabic numerals, the direction being so chosen as to given the lowest numbers possible to the side chains. When series of locants containing the same number of terms are compared term by term, that series is “lowest” which contains the lowest number on the occasion of the first difference (Lowest sum rule). This rule is applied irrespective of the nature of the side chains.
Examples :
Univalent branched radicals derived from hydrocarbon are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical containing the LPCC starting from the carbon atom with the free valence, this atom being numbered as 1.
The following names can be followed for the unsubstituted radicals only:
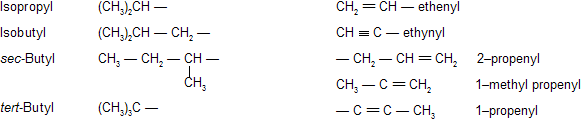
If two or more side chains of different nature are present, they are cited in alphabetical order and decided as follows
If two or more side chains of different nature are present, they are cited in alphabetical order and decided as follows
(i) The names of simple radicals are first alphabetized and the multiplying prefixes are then inserted.
Example :
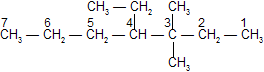
Ethyl is cited before methyl, thus 4-Ethyl-3, 3-dimethylheptane