Electrolytic Conductor: Which conducts electricity by undergoing decomposition where current is passed through them.
Factors affecting electrolyte conduction:
(1) Interionic attractions: depends on solute-solute interactions.
(2) Solvation of ions: depends on solute-solvent interactions.
(3) Viscosity solvent: depends upon solvent-solvent interactions.
Note: Electrolytic conduction
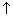
with
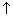
in temp.
Dumb Question: Why electrolyticconduction
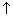
with
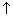
in temp ?
Ans: On increasing Temp., dissociation
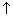
. So, conduction
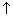
.
(4) Conc. of sol.: Higher conc. of sol., lessa is conduction. b/c if weak electrolyte, ionization is less & if strong electrolyte.
higher interaction at higher cond.
with dilution, conduction
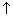
.
Conduction: Reciprocal of electrical resistance.
C =
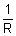
or a =
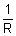
It unit is siemen (S) or ohm
-1Specific Conductivity:
R
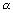
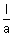
R =
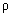
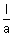
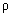
specific resistance.
Reciprocal of Resistivity c/d specific conductivity.
It is denoted by K
R =
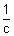
&
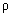
=
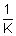
R =
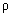
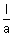
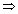
i
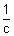
=
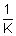
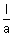
K = c x 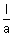 |
Units: Specific conductivity (K) =
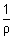
=
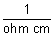
= ohm
-1 cm
-1 or
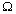 -1
-1 cm
-1 or
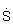
cm
-1 1 S cm
-1 = 100 Sm
-1Equivalent Conductivity: Conductance of all the ions produced from one gram equivalent of electrolyte dissolved in V cm
3 of sol. When distance b/w electrodes is 1 cm, & ares of electrode is so large that whole of sol. is contained b/w them. It is represented by
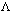 eq
eq.
RElationship b/w equivalent conductivity & specific conducvity:
If vol. of sol. containing 1 gm equivalent of electrolyte is V cm
3.
Eq. conductivity = Specific conductive x V
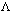 eq = Kv x V eq = Kv x V |
If conc. of sol. is c gm equivalent per litre, then,
1 gm eq. will be
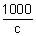
cm
3.
Dumb Question: Why C
eq = Normality ?
Ans: B/C normality is no. of gm. eq. of solute in 1 L of solution.
Units:
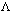 eq
eq = K x
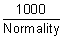
= ohm
-1cm
-1 x
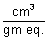
=
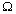 -1
-1 cm
2 eq
-1 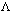 eq = eq = 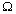 -1 cm2 eq-1 -1 cm2 eq-1 |
Molar Conductivity: Conductance of all ions produced from 1 mole of electrolyte dissolved in V cm
3 of sol. when electrodes are 1 cm apart & area of electrodes is so large that whole of sol. is contained b/w them. IT is represented by
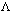 m
m.
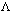 m
m = K
v x v
Units: ohm
-1 cm
-1 x
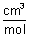
= ohm
-1 cm
2 mol
-1Specific Conductivity:
K = c x
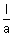
Specific conductivity = conductance x 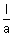 |
Illustration: A potential difference of 20V applied to ends of a column of
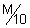
AgNO
3 sol., 4 cm in dia & 12 cm in length give current of 0.2 A. Calculate specific & molar conductance of solution.
Ans: By Ohm's law,
Resistance of sol. (R) =
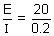
= 100
Area of x-section of column (a) =
r
2 = ... x (2)
2 cm
2 = 12.57 cm
2Length of column (distance b/w electrode), l = 12 cm
Conductivity (K) = c x
K =
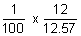
= 9.55 x 10
-3 S cm
-1 Molar conductivity (
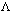 m
m) =
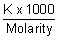
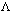 m
m =
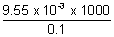
= 95.5 S cm
2 mol
-1Electrolytic conduction, eq. conductivity & molar conductivity
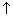
with dilution whereas specific conductivity of electrolytic sol.
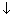
with dilution.
Dumb Question: Why this happen ?
Ans: Conductivity of sol. is to presence of ions in sol. Greater no. of ions, greater is conductance. As on dilution, more ions are produced in sol. So, conductance should
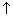
on dilution.
Specific conductivity
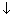
with dilution b/c no. of current carrying particles i.e. ions present per cm
3 ofsolution becomes less and less on dilution.
Conductance Behaviour of Strong electrolytes:
Molar conducting of strong electrolytes is found to vary as.
A
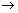
Constant.
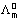
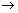
Molar conductivity at infinite dilution.
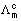
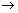
Molar conductivity at any conc.
This eq. hold goods at 1000 conc.
Note: (1) At higher conc., greater inter-ionic attractions retaral motion of ions & so, conductance falls with increasing concentrations.
(2) With dilution interionic attractions
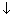
due to which conductance
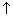
with dilution & max. at infinite dilution.