Electrochemical Cell: Device used to convert chemical energy produced in redox rxn. into electrical energy is c/d electrochemical cell.
Galvanic Cell:
Redox Rxn. b/w Zn & CuSO4:
Zn + CuSO
4 
ZnSO
4 + Cu(S)
Zn + Cu
2+ 
zn
2+ + Cu
Zn

Zn
2+ + 2e
- 
Cu (Reduction half rxn.)
Salt bridge & its functions: It is U-shaped tube containing conc. solution of inert electrolyte like KCl, KNO
3, K
2SO
4 etc.
Functions:
(1) To complete clectrical circuit by allowing ions to flow from one sol. to other without mixing oftwo solutions.
(2) To maintain electrical neutrality of solutions in two half cells.
Features:
(i) Zn electrode at which oxidation takes place is c/d anode & Cu electrode4 is c/d cathode.
(ii) Since electrons are produced at Zn electrode, this electrode is rich in electrons & pushes electrons into external circuit. So, it is c/d -ve pole. & copper electrode need electrons for reduction of Cu
2+ ions into Cu & pulls electrons from external circuit. So, acts as +ve pole.
(iii) Electrons flow from -ve pole to +ve pole in external circuit & current flow in opposite direction.
(iv) Wt ofCu rod will
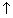
while that of An rod will
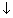
as cell words.
(v) In an increasing external opposing potential is applied. to cell, rxn continues as long as E
ext < E
cell. If E
ext = E
cell. rxn slops & IF E
ext > E
cell, now current flow in opposite direction & cell acts as electrolyte cell.
Redox Rxn. b/w Cu & AgNO3 sol. :
Cu + 2 Ag
+ 
Cu
2+ + 2Ag
Cu

Cu
2+ + 2e
- (Oxidation half rxn)
2 Ag
+ + 1e
- 
2Ag (Reduction half rxn)
Representation of Electrochemical Cell:
By convention, oxidation electrode is written on L.H.S. & reduction electrode on R.H.S. On L.H.S. writing symbol of metal I
st followed by symbol ogf ion with its conc. in bracket. On R.H.S., ion I
st along with its conc. followed by symbol of metal. Single vertical line for phase boundari & double vertical line for salt bridges.
Zn | Zn
2+(c
1) || Cu
2+ | Cu
oxidation Salt Reduction
occurs bridge occurs
Anode cathode
-ve pole +ve pole
E
0cell = E
0cathode - E
0anode =
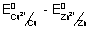
E
0cathode & E
0anode 
Reduction potential.
Electrode Potential: Electrical potential difference set up b/w metal & its ions in sol.
It is c/d oxidation potential if oxidation takes place at electrode. w.r.t. standard Hydrogen electrode & c/d reduction potential if reduction takes place.
If T = 298 K, Molar conc. is unity, then electrode potential is standard e;ectrode potential.
Hydrogen electrode: H
2 gas at 1 bar passed into 1 M HCl at 298 K & Pt electrodes is there
When in a cell, this H-electrode acts as anod, then
H
2 
2n
+ + 2e
- 
This give reduction potential of other electrode.
When H-electrode acts as cathode, then
2H
+ + 2e
- 
H
2 
This give oxidation potential of other electrode.
Note: Electrode potential of standard hydrogen electrode is zero at 297K.
Determination of standard electrode Potential of Zn/Zn2+ electrodeStandard electrode potential of
Zn/Zn
2+ electrode = 0.76 v But direction of current indicates. This is oxidation potential.
Similarly standard electrode potential of Cu/Cu
2+ electrode
= 0.34 v. & direction of current indicates that it is standard reduction potential.
Note: (1) Reduction Potential = - Oxidation Potential.
(2) It is imp. to mention all electrode potential as reduction potential.
Emf of Cell: Difference b/w electrode -potentials of two half cells c/d EMF.
EMF depends on (i) nature of reactions (ii) conc. of sol. in two half cells (iii) temp.
Diff b/w EMF & Potential difference:
(1) Emf. is p.d. b/w two electrodes of cell when no current is flowing in circuit but p.d. is diff. b/w electrode potentials of two electrodes under any condition.
(2) EMF is max. voltage obtainable from cell butp.d. is less than max. volatge of cell.
Electrochemical Series:
Reduction half rxn Reduction potential (v)
Li
+ + e
- 
Li - 3.05
K
+ + e
- 
K - 2.93
Ba
2+ + 2e
- 
Ba - 2.9
Cu
2+ + 2e
- 
Cu - 2.87
Na
+ + e
- 
Na - 2.71
Mg
2+ + 2e
- 
Mg - 2.37
Al
3+ + 3e
- 
Al - 1.66
Mn
2+ + 2e
- 
Mn - 1.18
2H
2O(l) + 2e
- 
H
2(g) + 2on
- - 0.83
Zn
2+ + 2e
- 
Zn - 0.76
Cr
3+ + 3e
- 
Cr
3+ - 0.74
Pb
2+ + 2e
- 
Pb - 0.13
2H
+ + 2e
- 
H
2 0.0
Sn
4+ + 2e
- 
Sn
2+ 0.013
Cu
2+ + 2e
- 
Cu 0.34
I
2 + 2e
- 
2I
- 0.53
F
2 - 12e
- 
2F
- 2.87
Applications:
(1) Calculation of standard EMF of electrochemical cells:
Illustration: Two half cells are Al
3+(ag)/Al & Mg
2+/Mg. Reduction potentials of these half cells are - 1.66 v & - 2.36 v respectively. Calculate cell potential.
Ans: EMF can be +ve if oxidation takes place at MG-electrode.
So, cell rxn.
3Mg + 2Al
3+ 
3Mg
2+ + 2Al
E
0cell =
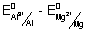
= - 1.66 - (- 2.36) = 0.7 v
(2) To predict whether a metal reacts with acid to give H2 gas:
M

M
+ + e
- (oxidation half rxn.)
H
+ + e
- 
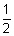
H
2 (Reduction half rxn.)
Those metal which have tendency to lse electrons i.e. -ve reduction potential will react with acid.
Lower reduction potential, higher is reactivity.