(3) To predict spontancity of redox rxn.:
For redox rxn. to be spotaneous, EMF of cell must be +ve.
Illustration: Cen Nickel vessel used to store CuSO
4 sol ?
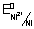
= - 0.25 v
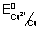
= 0.34 v
Ans: NI
2+/Ni || Cu
2+/Cu
E
0cell =
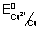
-
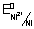
= 0.34 - (- 0.25) = 0.59 v
EMF comes out to be +ve. So, CuSO
4 will reacts with Ni. So, cannot store in nickel vessel.
Nerst's Equation:
M
n+ + ne
- 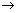
M
Nerst eq. as follows
E = E
0 -
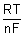
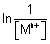
E
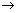
electrode potential at given conc. of M
n+ ions & Temp. T.
R
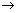
gas constant.
T
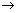
TEmp. in K.
F
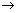
1 Faraday.
n
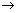
no. of electrons involved in electrode rxn.
For pure solids or liquids or gases, at 1 atm, molar conc. taken as unity.
i.e. [M) = 1
So, E = E
0 -
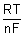
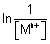
Putting value of R, T & F R = 8.314, T = 298 K, F = 965000 coulom
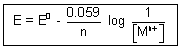
......................................... (i)
At 298 K Temp.
Nerts eq. for EMF of cell:
Zn(s) + Cu
2+(ag)
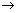
Zn
2+(ag) + Cu(s)
For Zn(s) | Zn
2+ (ag.) electrode,
Zn
2+(ag) + 2e
- 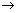
Zn(s)
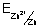
=
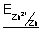
+
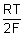
[Zn
2+(ag.)]
For Cu/Cu
2+ electrode
Cu
2+ + 2e
- 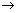
Cu(s)
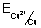
=
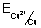
+
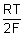
ln[Cu
2+(ag.)]
Since oxidation takes place at Zn & reduction takes place at Cu electrode.
E
cell = E
cathode - E
anode =
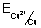
-
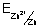
= {
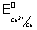
+
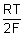
ln[Cu
2+(ag.)]} - {
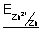
+
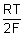
ln[Zn
2+(ag.)]}
= (
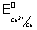
-
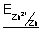
) +
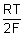
n
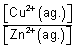
= E
0cell -
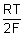
ln
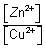
For cell rxn,
aA + bB
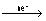
xX + yY
E
cell = E
0cell -
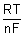
ln
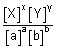
At 296 K
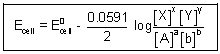 Illustration
Illustration: Calculate emf of cell
Cr|Cr
3+(0.1 M) || Fe
2+(0.01 M) | Fe
= - 0.75 v

= - 0.45 v
Ans: 2Cr + 3Fe
2+ 
2Cr
2+ + 3Fe n = 6
E
cell = E
0cell -
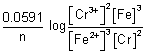
= (

-
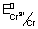
) -
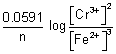
= (- 0.45 + 0.75 v) -
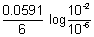
E
cell = 0.2606 v
Eqm. constant form Nerst Eq.:
Zn + Cu
2+ 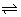
Zn
2+ + Cu
eqn. const =
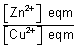
= KC
Eqn. occurs when E
cell = 0
0 = E
0cell -
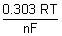
log
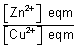
E
0cell =
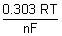
logK
cAt 296 k,
E0cell = 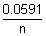 logKc logKc |
Illustration: Calculate eqm. constant for rxn.
Cu(s) + 2Ag
+ 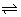
Cu
2+ + 2Ag
Ans: Here n = 2
E
0cell =
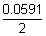
logK
c 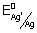
-
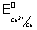
=
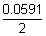
logK
c 0.8 - 0.34 =
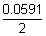
logK
c K
c = 3.688 x 10
15Dibb's Free Energy & Cell Potential:
-
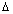
G
0 = nFE
0cellBut for rxn. at eqm
E
0cell =
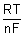
ln K
c 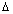
G
0 = - nF x
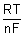
ln K
c 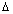 G0 = - 2.303 RT ln Kc G0 = - 2.303 RT ln Kc |
Illustration: Calculate standard free energy change
Zn(s)|Zn
2+ || Cu
2+(1M)|CU(s)
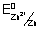
= - 0.76 v
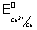
= + 0.34 v F = 96500 C mol
-1Also calculate eqm constant for rxn.
Ans: mrxn. n = 2
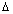
G
0 = - nFE
0cell E
0cell = 0.34 - (- 0.76) = 1.1 v
G
0 = - 2 x 96500 x 1.1 = - 212.3 KJ/mol
G
0 = - 2.303 RT logK
c - 212300 = - 2.303 xn 8.314 x 298 x logK
c K
c = 1.6 x 10
37