Easy Type
Q.1. Calculate quantity of electricity that will be required to liberate 710 g of Cl
2 gas by electrolysing a conc. sol. of NaCl what weight of NaOH & what volume o fH
2 at 27
0c & 1atm pressure i sobtained during process ?
Ans: W = ZQ = ZTt =
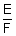
It =
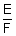
Q
2Cl
- 
Cl
2 + 2e
- 
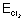
=
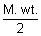
Q =
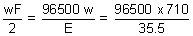
= 20 F
Q = 1930000 coulomb.
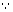
1 F gives 1 eg. or 40g NaOH
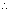
20 F gives 20 eg. or 40 x 20 g = 800 NaOH
Also
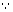
1 F gives 1g eg. or 1 g H
2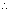
20 F gives 20 g eg. or 20 g H
2 PV =
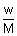
RT
1 x v =
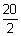
x 0.0821 x 300
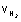
= 246.3 L
Q.2. Calculate volume of CL
2 at NTP produced during electrolysis of MgCl
2 which produces 6.5 g Mg. At wt. o fMG = 24.3.
Ans:
At Cathode: Mg
2+ + 2e
- 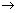
Mg
At Anode: 2Cl
- 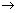
Cl
2 + 2e
-Eq. of Mg formed at cathode = Eq. of Cl
2 formed at anode
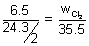
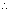
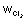
= 18.99 g
At NTP
PV =
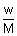
RT
1 x v =
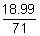
x 0.0821 x 273
Q.3. How long would it taice to deposit 100g ofAl for electrolytic cell containing Al
2O
3 using a current of 125 A ?
Ans: 2Al
3+ + 6e
- 
2Al
Eq. wt of Al =
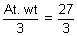
= 9
w = ZIt =
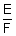
It
100 =
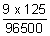
x t
t = 8577.77 second.
Q.4. 3 ampere current was passed through an ag. solution of unknown salt of Pd for 1hr 2.977g of Pd
n+ was deposited. atcathode. Find n(At. wt. of Pd = 106.4)
Ans: Pd
n+ + ne
- 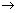
Pd
w =
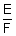
It
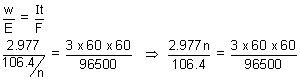
n = 4
Q.5. Electrolysis of a sol. of MnSO
4 in ag. H
2SO
4 is method of preparation of MnO
2.
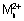
(ag.) + 2H
2O
MnO
2(s) + 2H
+(ag.) + H
1(g)
Passing a current of 27A for 24 hr. gives 1 Kg of MnO
2. What is value of current efficiency.
Ans: w =
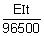
Mn
2+ 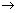
Mn
4+ + 2e
- 1000 =
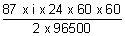
eq. wt. =
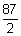
i = 25.6 A
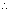
Current efficiency =
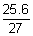
x 100 = 94.8 %
Q.6. How many gms. ofsilver could be plated out on serving tray by elctrolysis of sol. containing Ag in +1 oxidation state for period of 8 hr at current o f8.46 A ? What is area of tray if thichness of silver plating is 0.00254 cm ? Density of silver is 10.5 g/cm
3.
Ans: w
Ag =
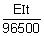
Eq wt. of Ag =
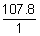
= 107.8
w
Ag =
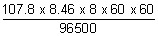
= 272.18 g
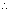
Volume of Ag deposited =
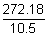
= 25.92 ml
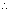
Surface area =
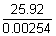
= 1.02 x 10
4 cm
2Q.7. Calculate
Zn|Zn
2+(ag) || Cu
2+(ag.)|Cu
min. conc. of Cu
2+ at which cell rxn.
Zn + Cu
2+(ag.)
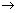
Zn
2+(ag.) + Cu will be spontaneous
if Zn
2+ is 1 M
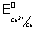
= 0.35 v
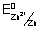
= - 0.76
Ans: At Anode Zn
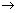
Zn
2+ + 2e
- At cathode Cu
2+ + 2e
- 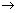
Cu

Zn + Cu
2+ 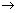
Zn
2+ + Cu
E
0cell =
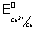
-
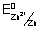
= E
0cathode - E
0anode E
0cell = 0.35 - (- 0.76) = 1.1 v
E
cell = E
0cell +
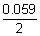
log
10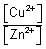
For rxn. to be spontaneous, E
cell = + ve
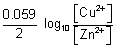
> - 1.11
log
10[Cu
2+] >
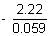
[Cu
2+] > 2.36 x 10
- 38 M
Q.8. Calculate PH of following half cells solution
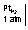
Hcl E = 0.25 v
Ans: H
2 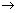
2H
+ + 2e
- 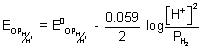
0.25 = 0 -
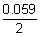
log[H
+]
2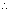
- log[
+] = 4.237
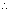
PH = 4.237
Q.9. Calculate eqm. constant for rxn:
Fe
2+ + Ce
4+ 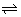
Fe
3+ + Ce
3+ 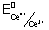
= 1.44 v
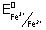
= 0.68 v
Ans: At eqm. E
cell = 0
E
cell = E
0cell -
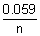
log
10K
c E
0cell =
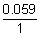
log
10K
c E
0cell = E
0cathode - E
0anode = 1.44 - 0.68 = 0.76 v
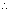
log
10K
c =
= 12.8814
K
c = 7.6 x 10
12