Ethers-Epoxides-2
Structure and bondangle in ethers and epoxides
Bonding in ethers is readily understood by comparing ethers with water and alcohols. Van der Waals strain involving alkyl groups causes the bond angle at oxygen to be larger in ether than alcohols, and larger in alcohols than in water. An extreme example is di-Isopropyl ether, where steric hindrance between the isopropyl groups is responsible for a dramatic increase in the C – O – C bond angle.
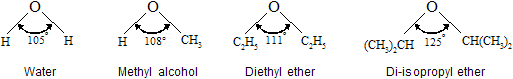
Carbon-oxygen bond distances are some what shorter than carbon-carbon bond distances. The C – O bond distances in dimethyl ether (141 pm) and methanol (142pm) are similar to one another, and both are shorter than the C – C bond distance in ethane (153 pm).
Insertion of an oxygen atom into a three-membered ring requires its bond angle to be seriously distorted form the normal tetrahedral value. In ethylene oxide, for example, the bond angle at oxygen is 61.5.
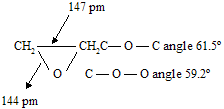
Thus epoxides, like cyclopropanes, are strained. They tend to undergo reactions that open the three-membered ring by cleavage of one of the carbon-oxygen bonds.
Physical properties
Physical properties of ethers are very similar with alkanes and alcohols. With respect to boiling point, ethers resemble alkanes more than alcohols due to less polarity than alcohol while; with respect to solubility in water, ethers resemble alcohols more than alkanes due to H–bonding with water.
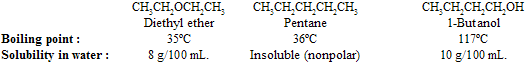
In general, the boiling point of alcohols are unusually high because of hydrogen bonding. Attractive forces are present in liquid phase of ethers and alkanes, but lack – OH groups so cannot take part in intermolecular hydrogen bonding, are much weaker, so their boiling points are less.
If we think about structure of ether, the presence of an oxygen atom permits ethers to participate in hydrogen bonding to water molecules. These attractive forces are responsible for solubility of ethers in water to approximately the same extent as comparably constituted alcohols. Alkanes cannot take part in hydrogen bonding with water.
Crown ethers
Cyclic compounds containing 4 or more ether linkages in a ring of 12 or more atoms. these compounds is known as crown ethers, because their molecular models are similar to crowns. Systematic nomenclature of crown ethers is actually a short hand description where by the word crown is followed by the total number of atoms in the ring and is followed by the number of oxygen atoms.
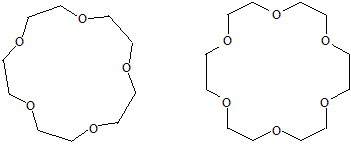
15-Crown-5 and 18-crown-6 are a cyclic pentamer and hexamer, respectively, of repeating -OCH2CH2 - units; they are polyethers based on ethylene glycol (HOCH2CH2OH) as the parent alcohol.
The metal ion-complexing nature of crown ethers are explain by their effects on the solubility and reactivity of salts in nonpolar solvents. Potassium fluoride is ionic and insoluble in benzene (covalent), but 0.05 M solutions can be prepared when 18-crown-6 is present. This high solubility of potassium fluoride in benzene is explain by the formation of a stable complex, cage like structure, is stabilized by ion-dipole forces between K+ and the six oxygen atoms of the crownether. Potassium ion, with an ionic radius of 265 pm, adjust completely with in the 260- to 320-pm inside cavity of 18-crown-6. Nonpolar CH2 groups dominate the outer side of the complex, make its polar interior, and permit the complex to soluble in nonpolar solvents such as covalent benzene. Every K+ that is carried into benzene brings a fluoride ion (F–) with it, resulting in a solution containing strongly complexed potassium ions and relatively unsolvated fluoride ions.
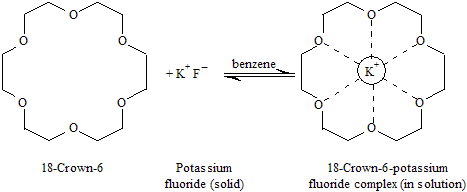
In polar matrix such as water and alcohols, fluoride ion is strongly solvated by hydrogen bonding and is neither very basic nor very nucleophilic. On the other part, the poorly solvated, or “naked,” fluoride ions that are present when potassium fluoride dissolves in benzene in the presence of a crown ether are better able to express their anionic reactivity. Thus, alkyl halides react with ionic potassium fluoride in covalent benzene solvent like 18-crown-6, easily gives a method of the preparation of different floride.