Ethers-Epoxides-3
(i) Willamson’s continous ethrification
Diethyl ether and many others, for example, are prepared by acid-catalyzed reaction of the corresponding alcohols, as we already studuied in alkenes, known as williamson contineous etherification.
In general, this method is limited to the preparation of symmetrical ethers in which both alkyl groups are primary. Isopropyl alcohol, however, is readily available at low cost and gives yields of diisopropyl ether high enough to justify making (CH3)2CHOCH(CH3)2 by this method on an industrial approach.
(ii) Williamson synthesis
A method of long standing for the preparation of ethers is the Williamson ether synthesis. Nucleophilic substitution of an alkyl halide by an alkoxide gives the carbon-oxygen bond of an ether.
Preparation of ethers by the Williamson ether synthesis is most successful when the alkyl halide is one that is reactive toward SN2 substitution. Methyl halides and primary alkyl halides are the best reactants (least reactive in elimination).
Secondary and tertiary alkyl halides are not suitable reactants, because they tend to react with alkoxide base by E2 elimination rather by SN2 substitution. Whether the alkoxide base is primary, secondary, or tertiary is not very important than the nature of the alkyl halide. Thus benzyl terbutyl ether is prepared in high yield from benzyl chloride, a primary chloride that is incapable of undergoing elimination, with potassium ter. butoxide (base).
The alternative synthetic route using the sodium salt of benzyl alcohol and an terbutyl halide would be much less effective, because of increased competition from elimination, as the alkyl halide becomes 3° than it easily undergo elimination.
We take some following example also, always prefer alkyl halide as primary.
The alternative combination, cyclohexyl bromide and sodium ethoxide, is not correct, because elimination will be the major product.
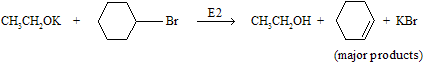
(iii) Oximercuration – demercuration of alkenes
Addition of ROH according to marknownikoff’s rule without any molecular rearrangement occurs.
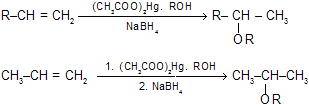 |