Ethers-Epoxides-7
(i) Ring–cleavage reactions of epoxides by base catalysed
Ethylene oxide very reactive substance, reacts rapidly and exothermically with anionic nucleophiles to yield 2-substituted derivatives of ethanol by cleaving the carbon-oxygen bond of the ring for example used for stepping up carbon atom in many family.
Nucleophilic ring opening of epoxides has many of the features of an SN2 reaction. Inversion of configuration is observed at the carbon at which substitution occurs.
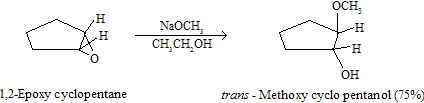
Unsymmetrical epoxides prefer to attack at the less substituted, less sterically hindered carbon of the ring in basic medium while opposite in acidic medium.
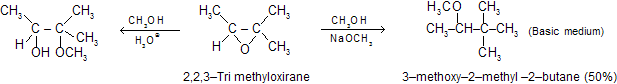
According to the mechanism the nucleophile attacks the less crowded carbon from the back side of the carbon-oxygen bond. Bond formation with the nucleophile followed by carbon-oxygen bond cleavage, and the strain in the three-membered ring is gone as it follows the opening of this ring from transition state. In this reaction initially an alkoxide anion is formed, which finally absorb a proton from the solvent to give a
Epoxides reduction takes place by lithium aluminum hydride or NaBH4 these are hydride donar so hydride is transferred to the less crowded carbon.
Remember here that epoxidation of an alkene, followed by lithium aluminum hydride reduction of epoxide, gives the same alcohol that would be given by same alkene by acid-catalyzed hydration or oxymercuration-demercuration.
(ii) Ring–cleavage reaction of epoxides by acid-catalyzed
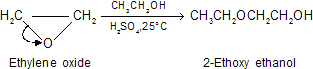
Here we discuss the preparation of ethylene glycol (HOCH2CH2OH) by hydrolysis of ethylene oxide in dilute sulfuric acid. There is a significant difference between the ring openings of epoxides the acid-catalyzed. Under conditions of acid catalysis, the species that is attacked by the nucleophile is not the epoxide itself, but rather its conjugated acid. The transition state for ring opening has a fair amount of carbocation character so the total system undergo strain. Breaking of the ring carbon-oxygen bond is more advanced than formation of the bond to the nucleophile.
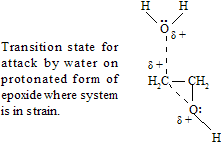
Because carbocation character develops at the transition state, substitution is prefer at the carbon that can better support a developing positive charge or stabilises intermediate. Thus, in comparison to the reaction epoxides with relatively basic medium, in which SN2- like attack is faster at the less crowded carbon of the epoxide ring, acid catalysis promotes just opposite at the position that bears the more number of alkyl groups for example.
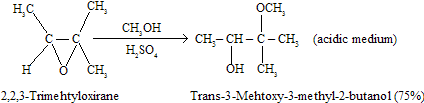
Here substitution proceeds with inversion of configuration for example back attack of nucleophile takes place so trans product are formed in these reactions.