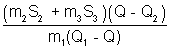Experimental Physics - 6
Description of searles Apparatus:
(a) Construction:-
Searles apparatus consists of two metal frames F1 and F2. Each frame has torsion head at the upper side and hook at the lower side. These frames are suspended from two wires AB and CD of same material, lengh and cross-section. The upper ends f the wires are screwed tightly in two torsin heads fixed in same rigid support.
A constant weight of 1 kilogram is suspended from the hook of the frame F2 attached to the auxiliary wire CD, which keeps the wire taut. A hanger H of 1 kilogram weight is suspended from the hook of the frame F1. The experimental wire AB can be loaded by slipping slotted weights on the hanger.
A spirit level rests horzontally with its one end hinged in the frame F2. The other end of the spirit level rests on the tip of the spherometer screw fitted in the frame F1. The spherometer screw can e rotated up and down along a vertical pitch scale marked in millimetre. The two frames are kept together by cross bars E1 and E2.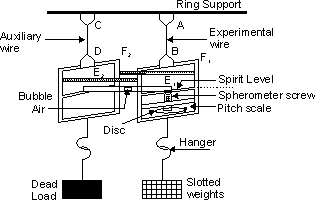
If a wire of length L and radius r be loaded by aweight mg and if l be the increase in length.
Then normal stress = 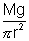
and longitudinal strain = 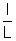
Hence, Young’s Modulus =  or
or 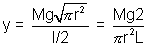
Knowing L and r, and finding L for known Mg, y can be calculated.
Dumb Question: What actually occure in this experiment ?
Ans:- Weights are placed in slotted portion of frame F1(with experimental wire). This causes wire AB to stretch, this is indicated by the Movement of spitit level away from centre (towards F2). Spherometer is used to bring back the spitit level at centre. The scale on spherometer indicates the amount by whichthe whole frame had moved or it gives the extension in wire. Hence y can be calculated.
Dumb Question:- Can the reading be immediately be taken after placing the weights.
Ans:- The process of elongation is not an instantaneous process and hence the reading should be taken few minutes later than placing the weights.
CALORIMETER:
Aim: FInd out the specific heat of a liquid calorimeter.
PRINCIPLE OF CALORIMETRY:- States that the total heat given by the objects equals the total heat received by the cold objects.
In a calorimeter at different temperature are made in contact with each other and thus heat rechange occurs.
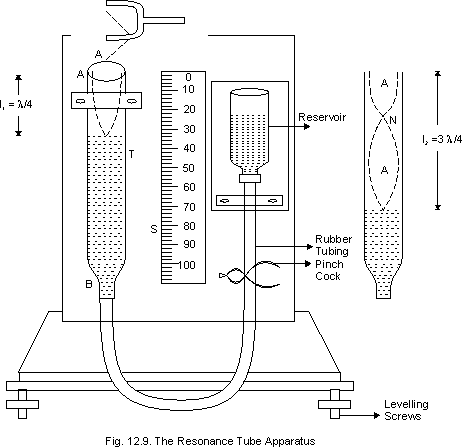
Determination of specific heat capacity in laboratiory: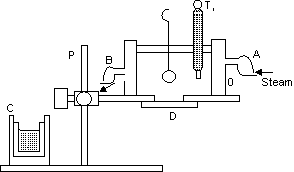
Figure shows Regnault’s apparatus to determine the specific heat capacity of asolid heavier than water, and insoluble in it. A wooden partion P separates a steam chamber O and a colorimeter C. The steam chamber O is a double walled cylinderical Vessel. Steam can be passed in the space between the two walls through an inlet A and it can escape out through an outlet B. The upper part of the vessel is closed by cork. The given solid may be suspended in the vessel by thread passing through the cork. A thermometer T1 is also inserted into the vessel to record the temperature of the solid. The steam chamber is kept on a wooden plateform with a removable wooden disc D closing the bottom hole f the chamber.
To start with, the Experimental solid (in the form of a ball block) is weighted and then suspended in the steam chamber. Steam prepared by bioiling water in aseparate boiler and is passed through the steam chamber. A calorimeter with a stirrer is weighted a sufficient amount of water is kept in it so that the solid may be completed immersed in it. The calorimeter is again weighted with water to get the mass of the water. The intial temperature of the water is noted.
When the temperature of the solid become constant(say for 15 min) the partition P is removed, the calorimeter is taken below the steam chamber, the wooden disc D is removed and the thread is cut of drop the solid in the calorimeter. The calorimeter is taken to its original place and is stirred. The maximum temperature of the mixture is noted.
Calculations:
Let the Mass of the solid = m
Mass of the calorimeter and the stirrer = m2
Mass of the water = m3
Specific heat capacity of the material of the calorimeter (and stirrer) = S2
Specific heat capacity of water = S3
Intial temperature of the solid = Q1
Intial temperature of the calorimeter , stirrer and water = Q2
Final temperature of the mixture = Q
We have,
heat lost by the solid = m1S1(Q1 - Q)
heat gained by the calorimeter (and stirren) = m2S2(Q - Q2)
and heat gained by water = m3S3(Q - Q2)
Assuming no loss of hea to be surrounding, the heat lost by the goes into the calorimeter, stirren and water. Thus
m1S1(Q1 - Q) = m2S2(Q - Q2) + m3S3(Q - Q2)
or S1 =