ABSOLUTE PRESSURE:
In equation (2), P is said to be the total pressure or absolute pressure.
GAUGE PRESSURE:
The difference between an absolute pressure and an atmospheric pressure is called the gauge pressure. Thus in equation (2) gauge pressure is rgh as P0 is atmospheric pressure because on surface of the vessel the pressure (P0) is solely due to atmosphere. Thus
Absolute Pressure - Atmospheric pressure = Gauge Pressure ------------ (3).
PASCAL LAW:
If the pressure in a liquid is changed at a particular point, the change is transmitted to the entire liquid without being diminished in magnitude.
Ex: Hydraulic lift is used to raise heavy loads such as car. It contains of two vertical cylinders A and B of different cross sectional areas A1 and A2. Pistons are fitted in both the cylinders as shown in fig (2).
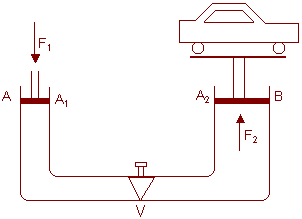
Fig (2)
The load is kept on a platform fixed with the piston of larger area. A liquid is filled in the equipment. A value V is filled in the horizontal tube which allows the liquid to go from A to B when pressed from the A side. The piston is pushed by a force F1. The pressure in the liquid increases every where by an amount F1/A1. Thus force on the larger piston in the upward direction is 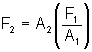 which raises the load upward.
which raises the load upward.
Thus if A2>>A1, even a small force F1 is able to generate a large force F2 which can raise the load.
Note:
There is no gain in terms of work. The work done by F1 is same as that by F2 if there is no dissipation due to friction etc. Thus
F1.d1=F2.d2
But F2>>F1 thus d1>>d2
i.e piston with greater area traverses a smaller upward distance as compared to the piston having smaller area that traverses larger downward distance.
Thus Pascal’s law is in consistence with the first law of Thermodynamics – “law of conservation of energy”.
In short
“With a hydraulic lever, a given force applied over a given distance can be transformed to a greater force applied over a smaller distance”
MEASURING PRESSURE:
a) The Mercury Barometer:
Figure (3) shows a very basic mercury barometer, a device used to measure the pressure of the atmosphere. The long glass tube is filled with mercury and inverted with its open end in a dish of mercury, as the figure shows. The space above the mercury column contains only mercury vapour, whose pressure is so small at ordinary temperature that it can be neglected.
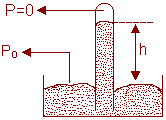
Fig (3)