fundamentals-of-organic-chemistry-1
Fundamentals of organic chemistry
Introduction
BREAKING OF A COVALENT BOND
Breaking of a covalent bond between two atoms can take place mainly in two alternative ways, viz. homolytic and heterolytic fissions depending upon the relative electronegativity of the two concerned atoms and medium of reaction.
(i) Homolytic fission takes place when the two atoms (say A and B) are usually of similar electronegativity
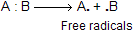
(ii) Heterolytic fission take place when the two atoms (A and B) are of different electronegativities. It may again take place in two different ways.
(a) When A is more electronegative than B
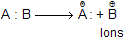
(b) When B is more electronegative than A
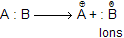
It is important to note that homolytic fission requires much less energy (e.g., 67.2 kcal/mole for C — Br bond in H3C — CH2 — Br into H3C —
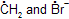 ) than the heterolytic fission (e.g., 183 kcal/mole for C — Br bond in CH3 — CH2 — Br into CH3 — CH2+ and Br-)
) than the heterolytic fission (e.g., 183 kcal/mole for C — Br bond in CH3 — CH2 — Br into CH3 — CH2+ and Br-)[1] Types of reactions
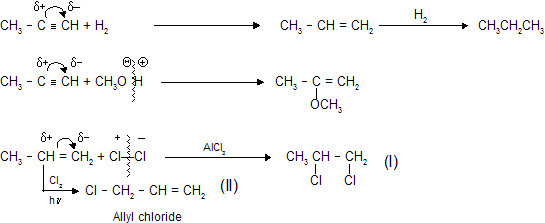
(i) Electrophilic addition reactions
Theses occur reaction in alkene and alkyne only where primary attack of electrophile takes place due to thick
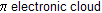 in between two carbon atoms
in between two carbon atoms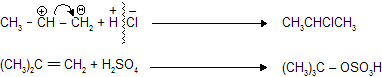
polarity in alkene and alkyne is created by + I and – I effect of different groups than attacking reagent add with opposite charge.
(II) reaction is not an example of electrophilic addition reaction because no addition occurs across the double bond while reaction (I) is an example of electrophilic addition reaction where addition occurs.

Due to dense
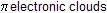 and delocalisation of electrons aromatic compounds undergo substitution reaction rather than addition reaction.
and delocalisation of electrons aromatic compounds undergo substitution reaction rather than addition reaction.