fundamentals-of-organic-chemistry-10
[8] Mechanism of elimination reactions
(a) Decarboxylation by sodalime (NaOH + CaO)
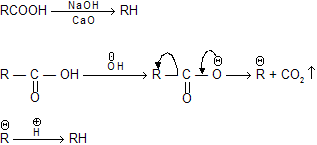
 acid undergo easily in decarboxylation even by heating due to highly stable intermediate carbanian.
acid undergo easily in decarboxylation even by heating due to highly stable intermediate carbanian.
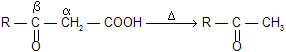
(b) Dehalogenation by Zinc dust
1,1,—dihalogen (gem) or 1,2–dihalogen (Vicinal) derivatives of the alkanes by reaction with zinc dust and methanol produces alkenes by loss of two halogen atoms (dehalogenation).
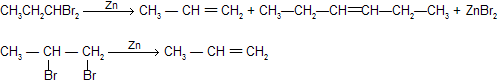
If sodium is used (instead of zinc) then 3 – hexane is mainly produced from 1,1–dibromo propane which is same as wurtz reaction.
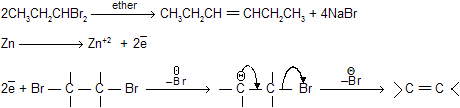
NaI in acetone can also be used instead of zinc dust for dehalogenation due to large size of iodine atom.
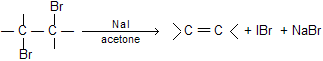
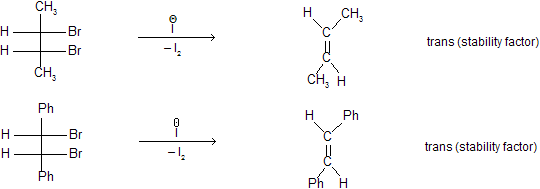
Addition (to the alkene) and elimination (from the dibromide) of the two bromine atoms are predominately trans so the reaction is stereoselective.
Elimination reactions are divided into three classes depending on the type of cleavage of two 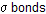
E 1 (Elimination) reaction
Step 1 : The C — L bond is broken heterolytically to form a carbocation (as in SN1 reaction)
Step 2 : Carbocation loses a proton from an adjacent carbon atom to form a 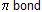 in presence of a nucleophile.
in presence of a nucleophile.
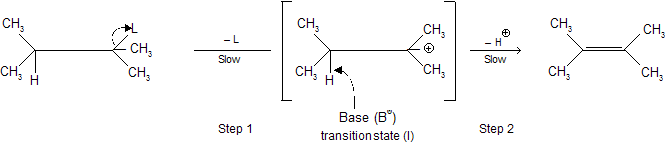
Step 1, in which  is lost is rate determining step hence we call it E1 (unimolecular elimination) reaction.
is lost is rate determining step hence we call it E1 (unimolecular elimination) reaction.
If base B– is added to the transition state then we call it nucleophilic substitution reaction whether a deprotonation or a substitution takes place on the transition state 1 depends on their relative rates but remember.
E1 reaction is favoured in compounds in which the leaving group is at a secondary or tertiary position while SN reaction is favoured in which leaving group is at primary carbon.
E1 — CB (Elimination) reaction
Step 1 : Consists of the removal of a proton, H+, by a base, generating a carbanion (2).
Step 2 : Carbanion (2) loses a leaving group to form alkene.
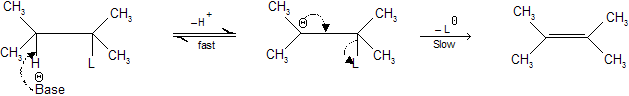
Because step 1 (deprotonation) is fast and reversible, the reaction rate is controlled by how fast the leaving group is lost from the carbanion (2) (conjugate base). The loss of L– from (2) in step (2) is rate determining step and is unimolecular. We call it E1 – CB (unimolecular conjugate base elimination) reaction.
E2 — (Elimination) Reaction
In this mechanism, two s bonds are broken and a 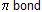 formation takes place simultaneously.
formation takes place simultaneously.
It is bimolecular since substrate and base are involved in the rate determining step. So we call it E2 (bimolecular elimination) reaction. Isotopic effect occurs because C – H bond breaking take place in a slow process.
E2 process does not proceed through an intermediate carbocation.

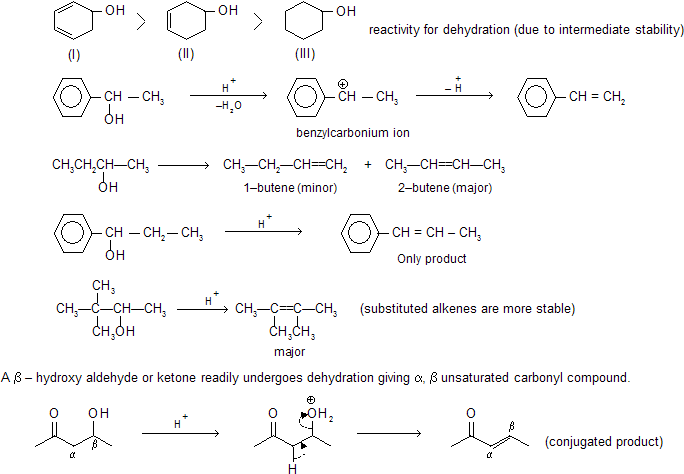
(c) Dehydration of Alcohols
Dehydration of alcohol in presence of an acid gives alkene by intermediate carbocation formation so rearrangement also occurs.
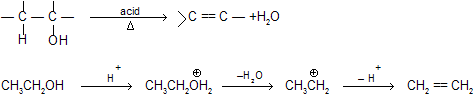
H2O is a better leaving group than OH– hence carbonium ion is formed easily by elimination of H2O by E1 mechanism. In case of 1° alcohol, loss of H2O forms less stable carbonium ion hence loss of water and deprotonation to form alkene takes place simultaneously and intermediate carbonium ion is not isolated. This will follow then E2 mechanism.
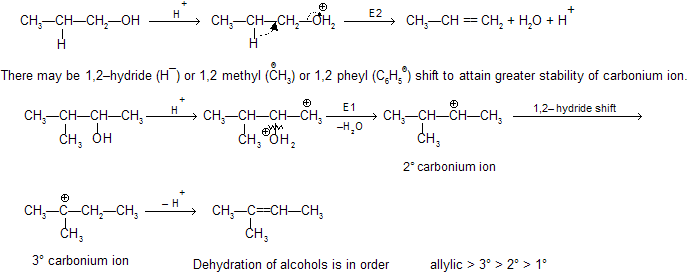
2° and 3° alcohol by E1 process and 1° alcohol by E2 process.
Alcohols leading to conjugated alkenes are more easily dehydrated than the alcohols leading to non–conjugated alkenes due to more stable intermediate carbonium so but –1–ene–2–ol is more easily dehydrated than –2–butanol.

Similarly