fundamentals-of-organic-chemistry-11
(d) Dehydrohalogenation (removal of HX)
Elimination of HX from alkyl halide is called dehydrohalogenation, takes place in presence of strong bases like alcoholic KOH or NaNH2 or potassium tertiary butoxide or metal alkoxides.
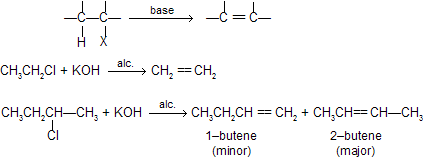
Dehydrohalogenation can follow go through by following mechanisms.
| (a) E1 — mechanism | (b) E2 — mechanism |
Reactivity of alkyl halide towards E2 or E1 elimination is the same 3° > 2° > 1°.
E1 — Dehydrohalogenation
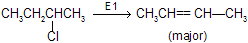
Step 1 : Loss of Cl produces a 2° carbonium ion in rate determining step
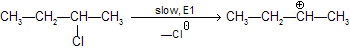
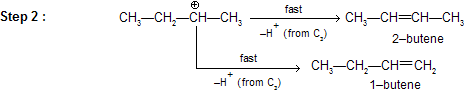
We know substituted alkenes are more stable, hence formation of 2–butene is preferred to 1–butene.

There can be 1, 2– hydride and 1,2–methyl shift to attain greater stability of the carbonium ion. This property of carbocation affects the regiochemistry of E1 elimination.
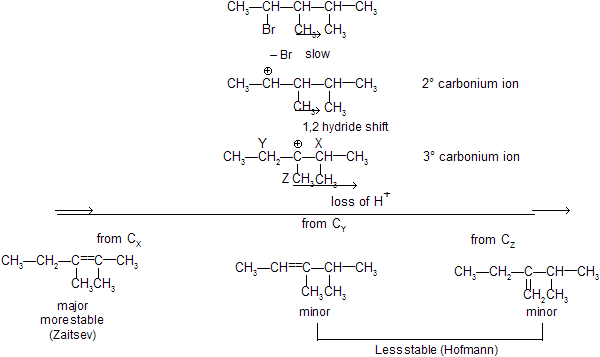
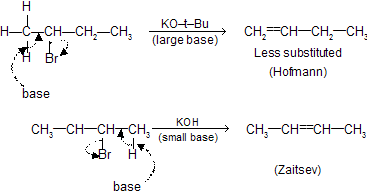
The formation of the less–substituted alkene in an elimination reaction is called as a ‘Hofmann Elimination’ and that of more–substituted alkene as a Zaitsev Elimination.
E2 – Dehydrohalogenation
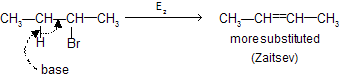
Loss of proton and leaving group (Br–) takes place simultaneously in presence of a base. Thus rate determining step, in which base and alkyl halide are involved, is bimolecular (E2).
Elimination of 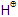 can also take place from C1 to give Hofmann product.
can also take place from C1 to give Hofmann product.
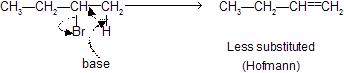
If base are large, due to steric hindrance, less substituted alkenes are preferred (Hofmann product). But if base is small, more substituted alkenes are preferred (Zaitsev product).
E2 elimination is stereospecific (Anti)
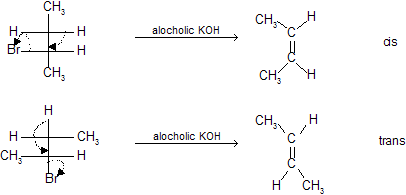
For trans–elimination leaving groups — H and — X must be as far apart as possible in anti relationship.