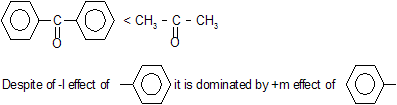fundamentals-of-organic-chemistry-5
[2] Inductive effect
Induction of charge due to less or more electronegative element is known as inductive effect. It occurs till four carbon atom and maximum at first carbon atom due to closeness impact.

Application of inductive effect
(a) Acidic strength of Carboxylic acid

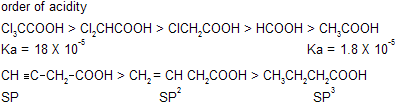
As the 'S' character increases electronegativity of atom also increases so that electro negativity order is sp >sp2 > sp3.

(b) Basic strength of amines
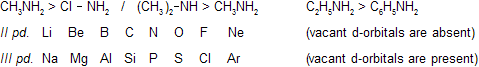
II pd. elements are more basic than III pd. elements because in II pd elements vacant d- orbitals are absent.
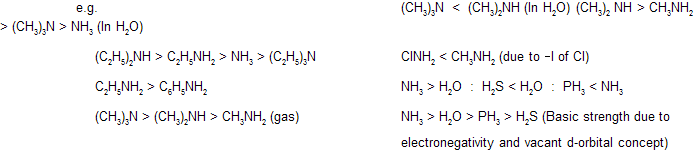
H2O < NH3 For same period elements consider electronegativity order. Less electro negative element is more basic.
R3N is less basic than R2NH in H2O because of the steric hinderance and solvation effect caused by the three bulky ‘R’ groups.
(c) Reactivity of Carbonyl Compounds
| HCHO > CH3CHO > CH3COCH3 |
carbonyl compounds give nucleophilic addition reactions where primary attack of nucleophile takes place.

As the size of alkyl group increases stearic hinderance comes into play thus reactivity decreases.