fundamentals-of-organic-chemistry-6
(d) Reactivity of alkyl halides
It can be deduced by reacting substance with Ag salt (AgNO3).
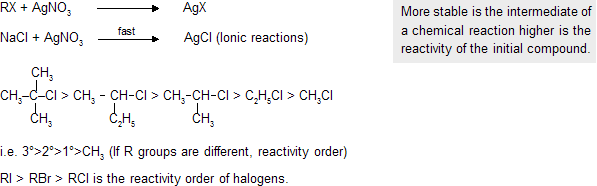
On the basis of bond dissociation energy RI is maximum reactive due to largest size of I atom and minimum bond dissociation energy.
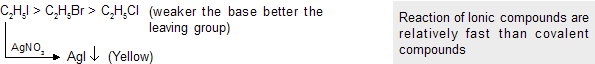
Free radical substitution reaction
These type of reactions are given by alkane, alkene and some aromatic compounds in the presence of sunlight or high temperature.
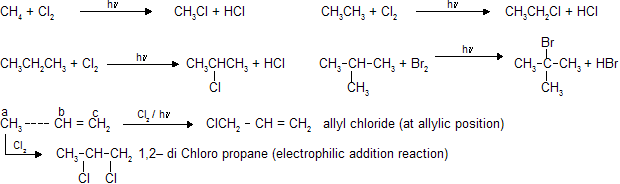
For free radical substitution in alkenes search for the allylic position and substitution takes place at this position because of intermediate stabilization through conjugation.
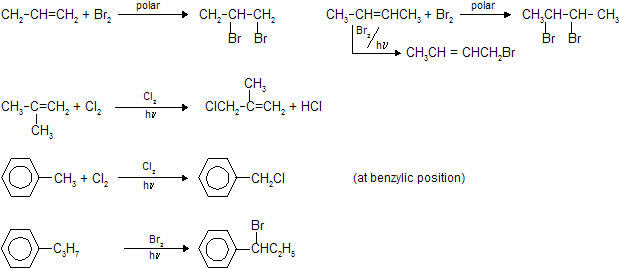
In toluene and their derivatives free radical substitution reaction occurs at benzylic position. If there is possibility of substitution at both allylic and benzylic position than substitution at benzylic position is given preference due to more intermediate stability.
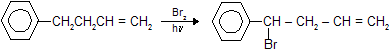
[3] Resonance
It is to be emphasized that the different contributing structures of a molecule differ only in the distribution of electrons and not in the arrangement of atoms, i.e., the position of atoms remains the same (different from tautomerism). Moreover, each canonical form must have same number of paired electrons, only their positions may differ. Usually, more are the number of canonical forms in a compound, more is its resonance energy, so more is the stability.
(i) Difference in stability of various canonical forms of a compound : All the canonical forms of a compound are not necessarily equally stable. One form may be more stable than the other and hence contributes more to the actual resonance hybrid. Following are the main factors governing the stability of a canonical structure :
1. Nonpolar structure is more stable than a polar structure.
2. More are the number of covalent bonds in a canonical structure, more is its stability.
3. In polar structures, that structure is more stable in which the negative and positive charges exist on the most electronegative and the most electropositive atoms respectively.
4. Out of the two canonical structures, the one with completed octet of various atoms (or duplet of hydrogen) is more stable even if the more electronegative atom has positive charge.
5. A canonical structure having an electron deficient positively charged atom is much less stable.
6. Also a structure in which like charge are on the atoms close to each other, is least stable.
(ii) Rules for writing resonance structures
(a) No real existence : Resonance structures exist only on paper. Resonance structures are useful because they allow us to describe molecules, radicals and ions for which a single lewis structure is inadequate. We write two or more lewis structures, calling them resonance structures of resonance contributors. We connect these structures by double headed arrows ![]() and we say that the real molecule, radical or ion is a hybrid of all of them.
and we say that the real molecule, radical or ion is a hybrid of all of them.
(b) In writing resonance structures we are only allowed to move electrons
Position of nuclei of the atoms must remain same in all resonance structures e.g.{
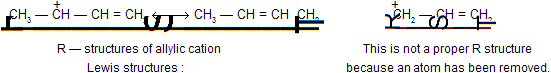
(c) All of the structures must be proper lewis structures
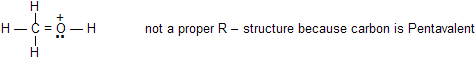
(d) Charge separation should be low since, to separate charge energy is required, therefore, structure in which opposite charges are separated have greater energy and hence are less stable.
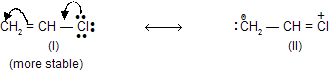
(iii) Resonance Energy
The difference in energy between the hybrid and the most stable canonical structure is called as Resonance energy.
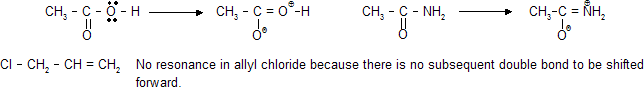
Any halogen or electronegative element always prefers a negative charge if there is both option of +ve and –ve charge
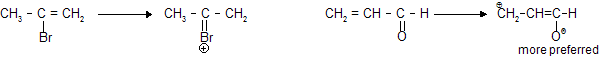
In the resonating structure of acryl aldehyde we prefers only 'O' with negative charge due to stability.
There is always two types of bonding one is localised and another one is delocalised which is responsible for resonating (contributing) structures.
Structural isomers are real molecules whose atoms are linked together in different ways to form different “skeletons” but resonating structures are not real. They are written whenever one electronic structure cannot adequately represent the actual structure.