fundamentals-of-organic-chemistry-7
(IV) Cross conjugation
Two systems conjugated with each other, a third system such that they are not conjugated with each other represent a cross conjugated system for example :

Prob. Account for the shorter C — O length in an ester compared with an anhydride. A degree of Cross – conjugation exists in the anhydride; this competition for electron decreases the delocalization of each carbonyl O and gives the C — O s bond less double – bond character.
A degree of Cross – conjugation exists in the anhydride; this competition for electron decreases the delocalization of each carbonyl O and gives the C — O s bond less double – bond character.
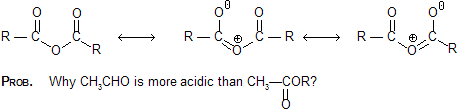
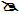 In the anion of CH3COOR, cross–conjugation with the O of OR competes with delocalization of the –ve charge delocalization from C and making the ester less acidic.
In the anion of CH3COOR, cross–conjugation with the O of OR competes with delocalization of the –ve charge delocalization from C and making the ester less acidic.
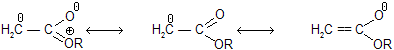
Prob. C6H5CONH2 is stronger base than CH3CONH2. Why? Due to Cross conjugation in C6H5CONH2 which increases its basic nature.
Due to Cross conjugation in C6H5CONH2 which increases its basic nature.
Aromatic compounds shows comparatively less exothermic enthalpies of combustion and hydrogenation as compare to aliphatic system. They also show a resistance towards addition reaction and easily undergo substitution (SE) reaction. In aromatic benzene type rings actually there is partial fixation of bonds. In this all the carbon atoms does not have same bond length and bond angle due to different bond order.
(V) Condition for aromaticity (one aspect of resonance)
(a) Which obey (4n + 2) Huckel rule
Huckel rule
(b) Planarity of molecule (sp2 hybridisation)
(c) Absence of sp3 hybridised atom in that portion where ring current is present.
(d) High resonating energy.
According to (4n + 2)  Huckel rule where n is whole number n = 0,1,2,3 ............
Huckel rule where n is whole number n = 0,1,2,3 ............ 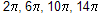 ....... aromatic system like cyclo propenyl cation is smallest aromatic ion. This rule also help in stability, acidic and basic nature of compounds.
....... aromatic system like cyclo propenyl cation is smallest aromatic ion. This rule also help in stability, acidic and basic nature of compounds.
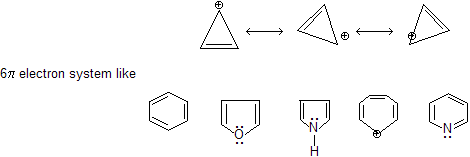
Prob. Why pyridine is more basic than pyrrole ? In pyridine lone pair cannot take part in resonance due to stable 6
In pyridine lone pair cannot take part in resonance due to stable 6  system while in case of pyrrole resonance occur by lone pair of electron, and it satisfy aromatic rule also.
system while in case of pyrrole resonance occur by lone pair of electron, and it satisfy aromatic rule also.
Prob. Cyclopropene is non aromatic while cyclo propenyl cation is a aromatic smallest cation. Why?
Prob. Cyclopropene is non aromatic while cyclo prop–2–ene–1–one is aromatic compound. Explain why?
Prob. Explain acidic strength order.
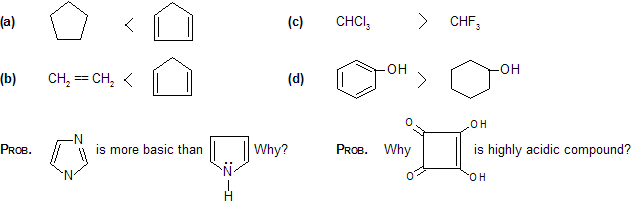
Prob. Why SiH3—OH more acidic than CH3OH?
Prob. Explain the acidic strength order
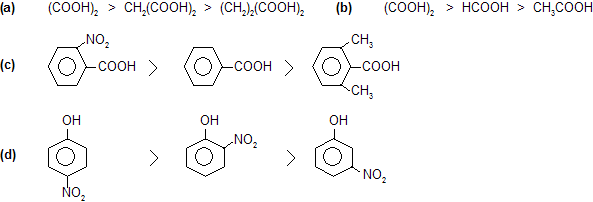
Prob. Explain basic strength order.
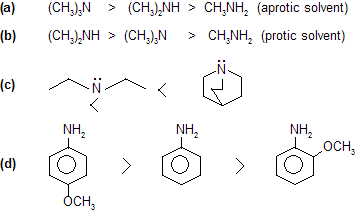
Prob. Write down the Lewis acid or base nature order
| (a) | NF3, NCl3, NBr3, NI3, (Lewis base) | Ans: | NI3 > NBr3 > NCl3 > NF3 |
| (b) | BF3, BCl3, BBr3, BI3, (Lewis Acid) | Ans: | BI3 > BBr3 > BCl3 > BF3 |
| (c) | B(OC2H5) and B(C2H5) (Lewis Acid) | Ans: | B(C2H5)3 > B(OC2H5)3 |
| (d) | (CH3)3N and (SiH3)3N. (Lewis base) | Ans: | (CH3)3N > (SiH3)3N |
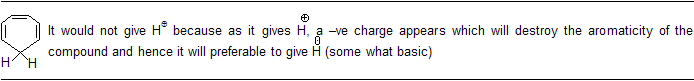
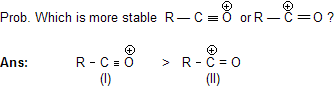
In this case as the electron system of oxygen is complete in first case so it is more stable but in (II) case this species does not contain complete octet of 'C' atom.
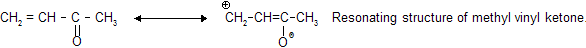
Prob. Basic nature: Explain basic strength order for CH3NH2>NH2CONH2>CH3CONH2
Ans: CH3NH2 is maximum basic due to absence of resonance.
Prob. Which is more reactive towards AgNO3 and why ?
| CH2 = CH - Cl < Cl CH2 - CH = CH2 |
In vinyl halide resonance occurs which develops double bond characters in C-Cl bond and reduces reactivity.

Stability of Carbocation
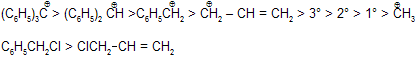
Benzylchloride is more reactive than allylchloride due to more stability of intermediate carbocation.
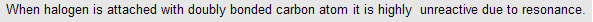
| For example | CH3 - CH = CH - Cl | Resonance |
| CH3 – C(Cl) = CH2 | Resonance | |
| CH2 = CH - Cl | Resonance |
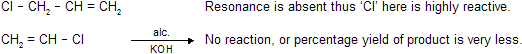
As ‘Cl’ is attached to doubly bonded carbon atom it is unreactive therefore NaNH2 (which is more powerful than alc KOH) is used for dehydrohalogenation.
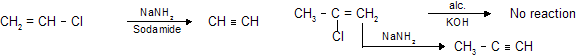
Conjugate diene C - C = C - C = C - C are those where alternate double bond are present. 1,3- Butadiene is also conjugated diene where resonance occurs and shows both 1,2- & 1-4 electrophilic addition reaction due to conjugation.
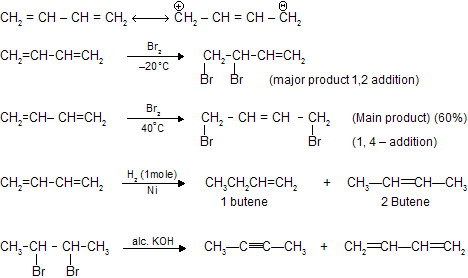
1,3 – Butadiene at low temperatures mainly forms 1,2 – product while at high temperatures forms 1,4 – product which is based on concept of thermodynamic control and chemical kinetics (discussed in next chapters).
Q.1. Explain basic character

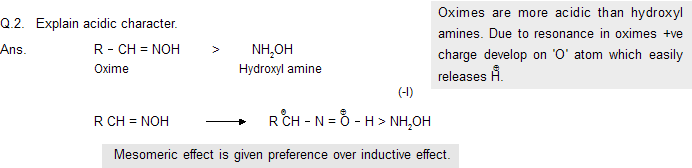
Q.3. Why guanidene NH2C (CH2)=NH is a strong base (pKb = 1)
| Q.4. Which one is more basic in pyridene and piperedene ? Ans. Piperdene, due to absence of resonance and sp3 'N' atom. | 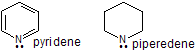 |
Q.5. Why chlorobenzene is less reactive than benzyl chloride ?
Ans . Due to resonance in chlorobenzene doubly bonded character in between C–Cl bond.