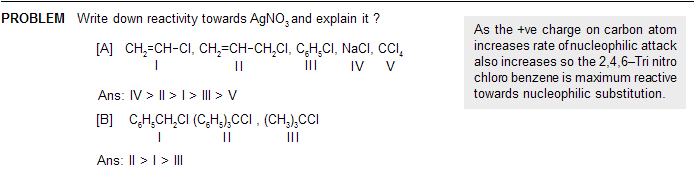fundamentals-of-organic-chemistry-8
(VI) Resonance in aromatic compounds
Phenol is acidic in nature due to resonance which develops +ve charge on ‘O’ atom and releases H in the solution. Due to negative charge at ortho and para position of benzene nucleus attack of electrophile takes place at o – & p – positions.
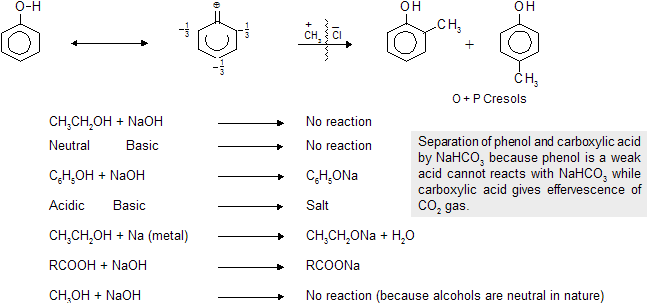
Prob. What is the separation test between following ?
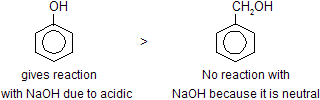
(a) Directive Influences of functional groups.
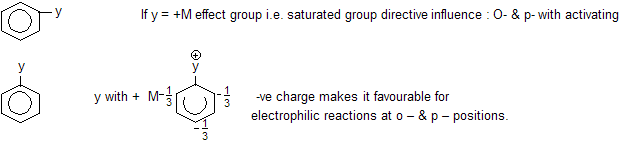
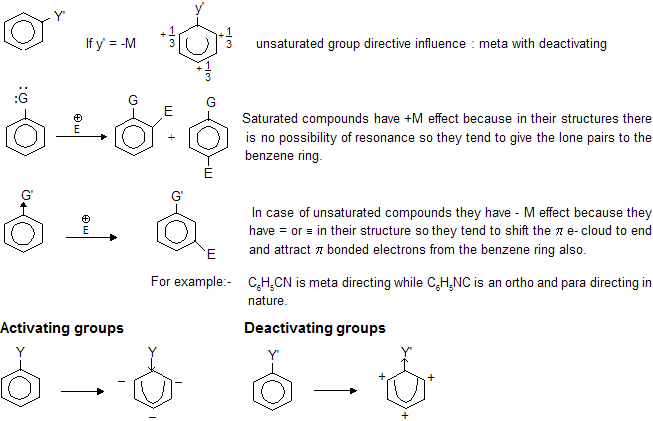
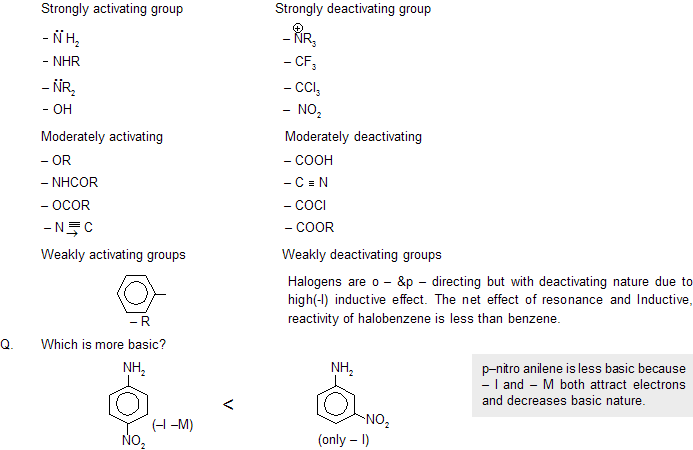
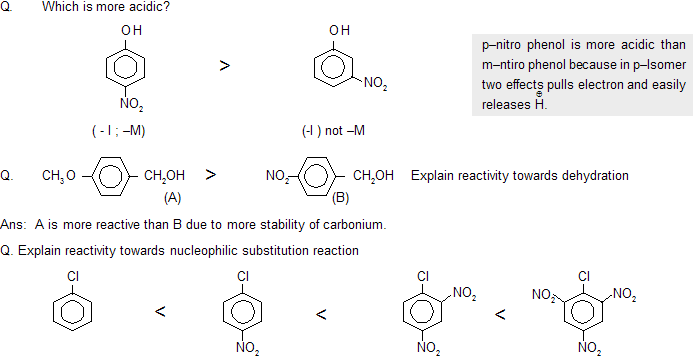
Ans: As the number of – NO2 group increases, positive charge on carbon atom increases so speed of attack of np also increases.