Gas Laws - 1
Measurement of ....:
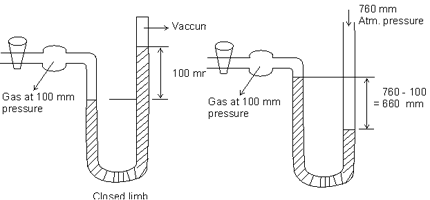
Instrument used for measurement of pressure of gases c/d manometer. They are of two type:
(1) One in which longer is closed which is used for measuring pressure less than atmospheric pressure.
(2) One in which longer limb is open which is used for measuring pressure greater than atmospheric pressure.
| 1 atm = 76 cm = 760 mm or 760 ... |
| 1 atm = 1.0132 bar 1 bar = 0.987 atm 1 Pa = 1 Nm-2 1 bar = 105 Pa |
Pressure: It is force per unit area.
Pressure of menometer =
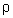 gh
ghDumb Question: How P =
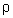 gh in manometer ?
gh in manometer ?Ans: Suppose height of mercury column = h cm
Area of cross-section of tube =
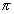 cm2
cm2 Vol. of mercury column = A x h cm3
Vol. of mercury column = A x h cm3Then mass of mercury column. 6 = A x h x
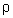 gm
gm Weight of mercury column = (A x h x
Weight of mercury column = (A x h x 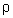 ) x g = Force
) x g = ForcePressure (P) =
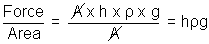
Illustration: Manometer is connected to a gas containing bulb. Open arm reads 43.7 cm whereas arm connected to bulb reads 15.6 cm. If barometer pressure is 743 mm mercury. What is pressure of gas in bulb.
Ans: Note: heights are measured always from bottom. Diff. of mercury levels in two arms = 43.7 - 15.6 = 28.1 cm. As level in limb connected to bulb is lower than that of open limb, this means pressure of gas is more than atm. pressure.
 Pressure of gas = Atm. pressure + Diff. in levels of mercury
Pressure of gas = Atm. pressure + Diff. in levels of mercury= 74.3 cm + 28.1 cm
= 102.4 cm
=
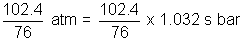
= 1.36 s bar
Measurement of Temperature:
| 0C = 5/9(F0 - 32) |
| K = 0C + 273.1 s |
C
 Celcius
CelciusF
 Farhenheit
FarhenheitK
 Kelvin
KelvinScals read same value ?
Ans: Suppose both read same value as 'x'
0C = 5/9(F - 32) Br /> x = 5/9(x - 32) => x = - 400C
II. Gas Laws:
(a) Boyle's Law: At constant temperatue, volume of a given mass of gas is inversely proportional to its pressure.
V


| => PV = constant |
Let P1 & V1 are initial pressure & volume of gas & P2 & V2 are final pressure & volume at constant temlerature.
P1V1 = P2V2  at constant temperature.
at constant temperature.
In other words.
at constant temperature gas density is directly proportional to pressure.
Dumb Question: How density is proportional to pressure ?
Ans: d = 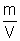
And PV = K, 
d = 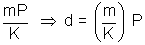
=> d  P
P
Question: gas at one atm. pressure is connected to evcuated bulb of 0.5 L capacity through stop-clock. On opening, pressure constant temperature. What is volume of bulb X ?
Ans: Suppose volume of bulb 'X' = VL
i.e. V1 = V & P1 = 1 atm
After connecting to bulb of 0.5 L
Volume = (V + 0.5) L
i.e. V2 = (V + 0.5) L, P2 = 570 mm = 570/760 atm.
By P1V1 = P2V2
1 X V =  x (v + 0.5)
x (v + 0.5)
On solving V = 1.5 L
(b) Charle's 0 Law: It states that pressure remaining constant, volume of given mass of gas increase or decrease by 1/293 of its volume at 00C for every 10C rise or fall in temperature.
Vt = V0 + 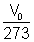 x t t
x t t  0C
0C
at constant pressure, volume of given mass of gas is directly proportional to its temperature in degrees kelvin.
V  T
T = constant
= constant 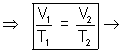 at constant pressure is directly proportional to its temperature in degrees kelvin.
at constant pressure is directly proportional to its temperature in degrees kelvin.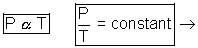 at constant volume.
at constant volume. at constant volume.
at constant volume.
Illustration: An open vessel contain 200 mg of air at 170C. What % of wt of air would be expelled if vessel is heated to 1170C.
Ans: Suppose volume of 200 mg of air at 170C = V ml
As pressure remains constant.
Dumb Question: Why pressure is constant ?
Ans: Because vessel is open & only atm. pressure is acting. V2 = 1.34 V
V2 = 1.34 V Volume of air expelled = 1.34 V - V = 0.34 V
Volume of air expelled = 1.34 V - V = 0.34 V
Mass of 1.34 V air at 1170C = 200mg
Mass of 0.34 V air at 1170C = 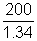 x 0.34 mg
x 0.34 mg Mass % of air expelled =
Mass % of air expelled =  x 100 = 25.37 %
x 100 = 25.37 %