Gas Laws - 3
VI. Kinetic Molecular Theory of Gases:
(a) Postulates:
(i) Molecules of gas are separated from each other by large distance so, actual volume of molecule is negligible as compared to total volume of gas.
(ii) Distance of separation between molecules are so large that of attraction are negligible.
(iii) Molecules are perfectly elastic. So, no wastage ……. energy during collision.
to absolute temperature of gas.
Kinetic Gas Equation
PV = 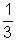 mnu2
mnu2
P  Pressure exerted by gas.
Pressure exerted by gas.
v  Volume of gas
Volume of gas
m  Mass of each molecule of gas
Mass of each molecule of gas
n 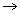 Total no. of molecules
Total no. of molecules
u 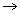 Root mean square speed of gas
Root mean square speed of gas
For 1 mole, m x n = mass of 1 mole
= M 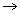 Molar mass in gm
Molar mass in gm
PV = 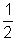 MV2
MV2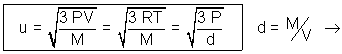 densityof gas.
densityof gas.
Most Probable Speed: This is speed possessed by max. fraction of molecules.
RMS Speed: It is square root of mean of square of speeds of molecules.
RMS = 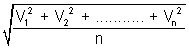
Average speed: It is average of different speeds of all molecule.
Average speed = 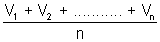
RMS Speed: U = 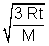
Most Probable Speed: 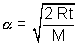
Average Speed: V = 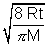
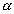 : v : u = 1 : 1.128 : 1.224 : v : u = 1 : 1.128 : 1.224 |
PV =
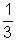 mnu2 =
mnu2 = 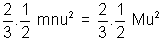 (mn = M
(mn = M 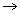 Total mass of gas)
Total mass of gas)But
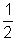 Mu2 = K.E. of gas
Mu2 = K.E. of gas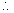 PV =
PV = 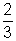 K.E. ............................................... (i)
K.E. ............................................... (i)K.E. Absolute Temperature (T)
K.E. = KT
Putting in eq.(i)
PV =
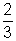 KT
KTAs
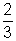 K is constant. So, if T is constant,
K is constant. So, if T is constant, 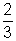 KT will be constant.
KT will be constant.| PV = constant |
(Boyle's law)
(c) Charle's law:
PV =
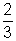 KT
KT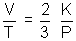
If P is constant, then
 Charle's law
Charle's law(d) Relation between Average Kinetic Energy & Absolute Temperature Kinetic Gas Equations:
According to kinetic gas equation
Pv =
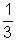 mnv2 ................................................ (i)
mnv2 ................................................ (i)Pv =
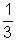 Mv2 (M = m x n) .................................. (ii)
Mv2 (M = m x n) .................................. (ii)Pv =
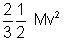
Pv =
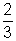 K.E.
K.E.K.E. =
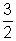 Pv for 1 mole of gas ................................... (iii)
Pv for 1 mole of gas ................................... (iii)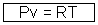
Putting this in eq. (iii)
K.E. =
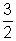 RT for 1 mole
RT for 1 moleAvg. K.E. of 1 molecule =
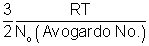
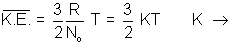 Boltzmann constant.
Boltzmann constant.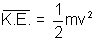
i.e.
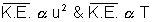
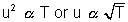
Illustration: At 270C, a gas contains 10 molecules travelling with speed of 4 m/s, 20 molecules travelling with speed of 5 m/s & 40 molecules travelling with speed 8 m/s. Calculate Avg. speed, RMS speed & Most probable speed & Molarmass of gas ?
Ans: Avg. speed (uav) =
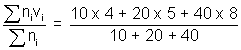 = 6.56
= 6.56RMS (vrms) =
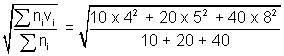 = 6.78
= 6.78Most probable speed = 0.816 x vrms = 0.816 x 6.78 = 5.53 m/s
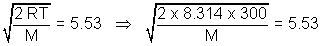
M =
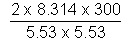 = 163.1 amv
= 163.1 amv