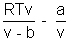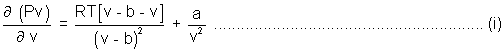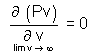Gas Laws - 4
Under all temperature & pressure.
No gas is ideal but they are real gases which obeys ideal gas eq. at low pressure & high temperature.
Ideal gas is characterised by following postulates:
(1) Ideal gas cannot be liquefied.
(2) There is no force of attraction b/w gas molecule.
(3) Volume of ideal gas molecule is negligible as compared to containce
For ideal gas
Compressibility factor z = 1
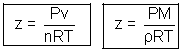
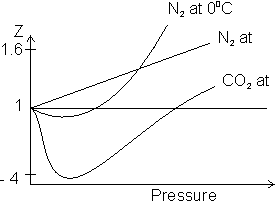
For real gas z
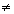 1.
1.z > 1 (Volume of gas is relatively more dominant)
It shows +ve deviation. This implies gas is less compressible.
z < 1 (Force of attraction of gas molecule is relatively more dominant)
It shows -ve deviation. This implies gas is more compressible.
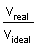
z =
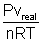 ................................................ (i)
................................................ (i)If gas shows ideal behaviour
Pvideal = nRT videal =
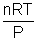
Substituting in (i)
z =
into account, force of attraction among molecule as nell as volume of gaseous molecule.
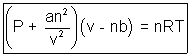
Derivation:
Correction for volume: Suppose volume occupied by gas molecules is v. When molecules are moving their effective is 4 times factual volume i.e. 4v.
b = 4v (excluded volume)
corrected volume = (v - nb) for n moles
Dumb Question: Why effective volume is 4 times of actual volume ?
Ans:
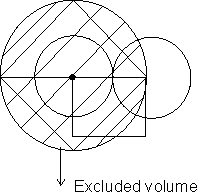
Excluded volume of 2 molecules is sphere of radius of 2R where R radius of ...
Excluded volume for two molecule
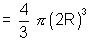
Excluded volume for one molecule ?
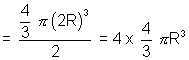
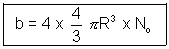
Constant 'a'
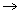 measures force of attraction. Greater value 'a', higher intermolecular force of attraction.
measures force of attraction. Greater value 'a', higher intermolecular force of attraction.Dumb Question: How unit of 'a' = atm L mol ?
Ans: P =
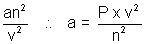
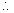 a = atm2L mol-2
a = atm2L mol-2Units of 'b' = L mol-1
Different forms of Vander Waal Equation:
(i) At very low pressure: v is very large. Hence, correction term a/v2 is negligible correction term 'b' is also negligible. Now Equation reduced to
| Pv = nRt |
That's why real gas behave like ideal gas at very low pressure.
(ii) At mederate pressure:V decreases. Hence a/v2 increases & cannot neglected but b is negligible because volume is still high.
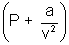 v = RT (for 1 mole)
v = RT (for 1 mole)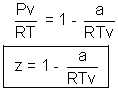
(iii) At high pressure: v is so small so b cannot be neglected. Factor a/v2 is no doubt large but as P is very high, a/v2 can be neglected.
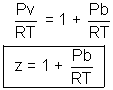
(iv) At high temperature: v is very large (at constant pressure). So, that both correction factors are negligible. (a/v2 & b)
| PV = RT |
So, at high temperature, real gases behave like ideal gas.
*Tip: Gases tend to behave ideally at high temperature and low pressure and non ideally at low temperature and high pressure.
Illustration: Calculate pressure exerted by 110g of CO2 in vessel of 2L at 270C. Given that Vander Waal's constants are a = 3.59 L2 atm mol-2 & b = 0.0427 L mol-1.
Ans: According to Vander Waal's Equation
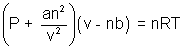
n =
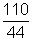 = 2.5 mol.
= 2.5 mol.P =
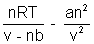
P =
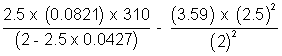
= (33.61 - 5.61) atm
= 28 atm
Shows ideal gas behaviour.
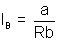
Mathematically defined.
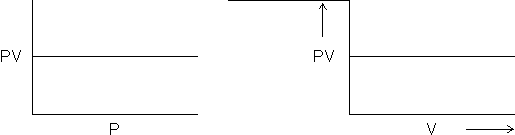
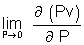 = 0
= 0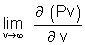 = 0
= 0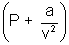 (v - b) = RT (For 1 mole)
(v - b) = RT (For 1 mole)P =
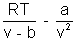
Pv =