Gas Laws - 6
Question: & 0.0566 L mol. Calculate a, b & R
Ans: Given TC = 374 + 273 = 647 K, PC = 218 atm
VC = 0.0566 L mol-1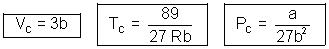
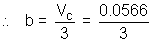 = 0.0189 L mol-1
= 0.0189 L mol-1
a = 3PCVC2 = 3 x 218 x (0.0566)2 = 2.095 L2 atm mol-2
R = 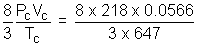 = 0.05086 L atm K-1mol-1
= 0.05086 L atm K-1mol-1
Medium Type:
Q1. A large flask filled with stop cock is evacuated & weight its mass is found to be 134.567 g. It is then filled to pressure 735 mm at 310C with a gas of unknown molecular mass & then reweighed; its mass is 137.456 g. Flask is then filled with H2O & weighed again, its mass is now 1067.9 gm. Calculate molar mass of gas if gas is ideal.
Ans: Mass of meter filling flask = (1067.9 - 134.567)g
= 933.333 g Volume of flask = Volume of meter = 933.333 ml
Volume of flask = Volume of meter = 933.333 ml
Dumb Quetion: Why volume of meter is 933.333 ml ?
Ans: Because density of meter is 1 g/cm3
P = 735 mm, T = 273 + 31 = 304 K, V = 933.33
By PV = nRT
n = 0.036 mol
Mass of 0.036 mol of gas = (137.456 - 134.567) g
= 2.889 g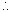 Mass of 1 mol of gas =
Mass of 1 mol of gas = 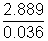 = 80.25 g
= 80.25 g
Q2. Avg. speed at T1K & Most probable speed at T2K of CO2 gas is 9 x 104 cm/s. Calculate value of T1 & T2.
Ans: Avg. speed = 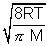 Most probable speed =
Most probable speed = 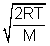
Avg. speed at T1K = MP speed at T2K for CO2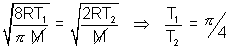 ……………………………….. (i)
……………………………….. (i)
For CO2 UmP = 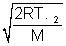 = 9 x 104
= 9 x 104
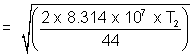 = 9 x 104
= 9 x 104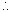 T2 = 2143.37 K
T2 = 2143.37 K
By eq. (i) T1 = 1684 K
Q3. Mass of molecule A is twice mass of molecule B. RMS speed of A is twice rms speed of B. If two samples of A & B contains same no. molecules. What will be ratio of P of two samples in separate containes of equal volume.
Ans: Given, mA = 2mB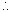 Mol. wt. of A = 2 x Mol. wt. of B
Mol. wt. of A = 2 x Mol. wt. of B
Given Urms of A = 2 Urms of B
For gas A PAVA = 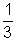 MA u2rms A
MA u2rms A
Fr gas B PBVB = 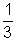 MB u2rms B
MB u2rms B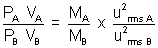
Given VA = VB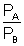 = 2 x (2)2 = 8
= 2 x (2)2 = 8
| PA = 8 PB |
Q4. One way of writing eq. of state of writing eq. of state of real gases is PV = RT[1 + B/V + ..........] where B is constant. Derive expression for B in terms of Vander Waal's constant a & b ?
Ans: According to Vander Waal's Equation
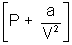 [V - b] = RT
[V - b] = RTP =
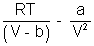
Multiply by V
PV =
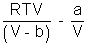
PV = RT
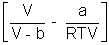
PV = RT
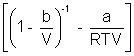
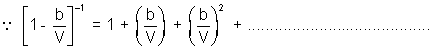
PV = RT
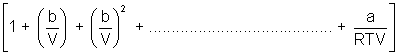
On comparing
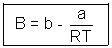
Q5. A glass capillary tube scaled at both ends is 100 cm long. It lies horizontally with middle 10 cm containing Hg. Two ends are equal in length contain air at 270C & pressure > 6 cm od Hg. Tube is kept in horizontal position, the one end is at 00C & other end is at 1270C. Calculate length of air column & pressure which is at 00C.
Ans:
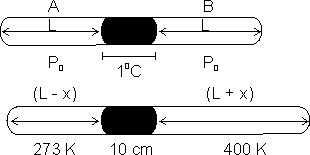
2 L + 10 = 100
=> L = 45 cm
Let initial pressure be Po atm on each side. When one end is cooled & other heated, expansion of gas takes place at hoter end till pressure of two sides becomes same.
i.e. P1 = P2 = P
For end A.
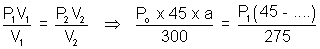 .......................... (i)
.......................... (i)a
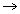 area of x - section of tube.
area of x - section of tube.For end B,
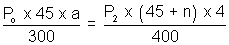 ................................. (ii)
................................. (ii)Equating. P1 = P2
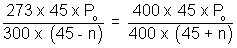
x = 8.49 cm
P1 =
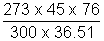 = 82.25 cm of Hg
= 82.25 cm of HgHard Question:
Q. An LPG cylinder weighs 14.8 Kg when empty, when full, it weighs 29 Kg & shows pressure of 2.5 atm. During use at 270C weight of full cylinder reduced to 23.2 Kg. Fid out volume of gas in m3 used up at normal condition & final pressure inside cylinde. Assume LPG to be n-butane.
Ans: Wt: of butane in cylinder = 29 - 14.8 = 14.2 Kg = 14.2 x 103
P = 2.5 atm, T = 300 K, Mol. wt. of butane = 58
PV =
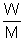 RT
RT2.5 x V =
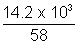 x 0.0821 x 300
x 0.0821 x 300V = 2.4 x 103 L = 2.4 m3
Now, wt. of as left after use = 23.2 - 14.8 = 8.4 x 13 g
Volume remains constant
PV =
 RT
RTP x 2.4 x 103 =
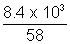 x 0.0821 x 300
x 0.0821 x 300wt. of gas given out = 29 - 23.2 = 5.8 Kg
= 5.8 x 103 g
Volume of gas given out
1 x V =
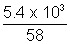 x 0.0821 x 300
x 0.0821 x 300 V = 2.4 x 103 L = 2.4 m3
V = 2.4 x 103 L = 2.4 m3