General Series - Chemistry Fact Sheet - 3
General Series - Chemistry Fact Sheet - 3
| 1. | 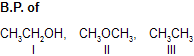 | There is intermolecular H-bonding I. III has weak force of attraction and is most volatile. | |
| 2. | B.P. of o, m, p-nitro phenol | Intramolecular H-bonding in o-isomer makes it more volatile. | |
| 3. | Reactivity of ... with Tollen’s reagent | —CHO group is easily oxidised compared to keto group due to redusing hydrogen. | |
| 4. | Reactivity of ... with Fehling’s solution | —do— | |
| 5. | Extent of hydration of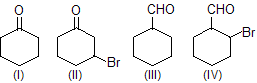 | Aldehydes are more hydrated than ketones. Halide makes C of carbonyl group more electropositive. | |
| 6. | Electrophilic nature of ........ for nucleophilic attack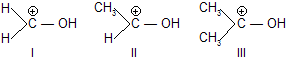 | CH3 group decreases +ve charge on C hence nucleophilic attack. | |
| 7. | Reactivity of isomeric 1°, 2°, 3° butyl halide towards elimination (E1 or E2) | due to stability of intermediate carbocation | |
| 8. | Dehydration of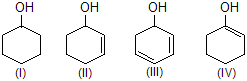 | Alcohol leading to increase in conjugation due to dehydration is more easily dehydration is more easily dehydrated. IV is vinylic, hence least. | |
| 9. | Stability of | ||
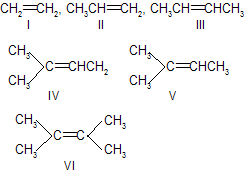 | < V < VI | Substituted alkenes are more stable.More the alkyl groups are attached to the doubly bonded carbon atom more is the stability. | |
| 10. | Stability of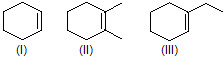 | II is more substituted than III (More hyperconjugation more stability) | |
| 11. | Stability of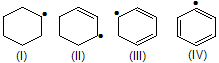 | IV is vinylic while in conjugative, II allylic. | |
| 12. | Stability of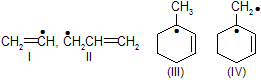 | III is 3° allylic and II is 1° allylic | |
| 13. | Dehydration of | More the stability of intermediate, greater the reactivity of chemical reaction. | |
| 14. | Boiling points of | I, II have H-bonding but electronegativity of O > N hence H-bonding in II > I | |
| 15. | Formation of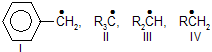 | (easiest I) | greater the stability, easier the formation of perticular species. |
| 16. | Reactivity of C—H bond (abstraction of H) | ||
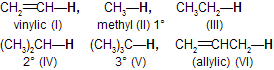 | < V < VI | Vinyl < methyl 1° < 2° < 3° < allylic | |
| 17. | Leaving nature (tendency) of ... in SN reaction.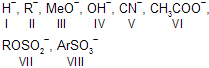 | < V < VI < VII < VIII | If acid is strong, its conjugate base is weak and greater the leaving tendency. |
| 18. | Rate of esterification of the following acids with MeOH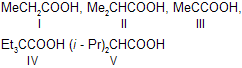 | > V | As the size of the substituents on the |
| 19. | Relative reactivity of ... with electrophile in SE reaction | ||
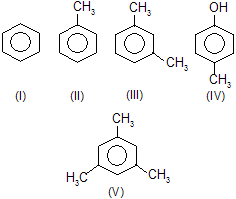 | > III > V | —CH3 is o-, p-directing and responsible for activation. | |
| 20. | Relative reactivity of these compunds with electrophile inSE reaction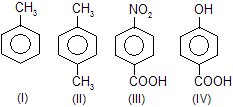 | —CH3 is o-, p-directing due to activation while —COOH is m-directing and deactivating group. | |
| 21. | Relative reactivity of ... with electrophile in SE reaction.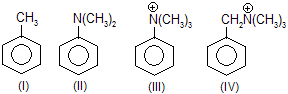 | As the number of sp3 hybridised C atoms separating the ring from the positively charged substituent increases, deactivating effect decreases due to less electronegativity. | |
| 22. | Activating effects of the following o, p-directors.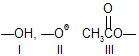 | ||
| 23. | Relative reactivity of ... towards SN1 reaction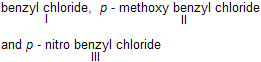 | Intermediates are benzylic cations. So CH3O(electron repelling) gives greater stability through delocalisation while NO2 (electron attracting) decreases stability. | |
| 24. | Relative reactivity of ... towards SN1 and SN2 reaction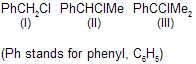 | II < II < I | SN1 : 1° < 2° < 3° alkyl halide SN2 : 3° < 2° < 1° alkyl halide |
| 25. | Relative reactivity of ... with E+ (electrophile) in SE reaction. | —NO2 deactivates benzene ring for SE | |
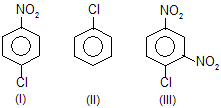 | |||
| 26. | Order of SN2 reactivity of alkoxide nucleophiles | ||
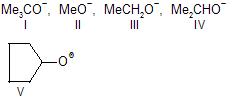 | < II | SN2 reactivity is suseptible to steric hindrance by the nucleophile as well as by the size of alkyl group. |