Isomerim-2
The two forms are readily interconvertible by acid or base catalysts, and under ordinary conditions surface of the glass is sufficient to catalyse the interconversion. The exact composition of the equilibrium depends upon the nature of the compound, solvent, temperature, etc. The conversion of a keto form into enol from is known as enolisation. The two forms of acetoacetic ester have been isolated under suitable conditions.
Keto-enol tautomerism in acetoacetic ester is proved by the fact that under ordinary conditions the compound gives the properties of the ketonic group as well as that of the enolic group.

Note that in all the examples of keto-enol tautomerism the two isomeric forms are interconvertible by the migration of a proton from one atom (carbon) to the other with the simultaneous shifting of bonds.
Remember that keto-enol tautomerism is possible only in those aldehydes and ketones which have at least one a -hydrogen atom which can convert the ketonic group to the enolic group. Examine the following compounds.
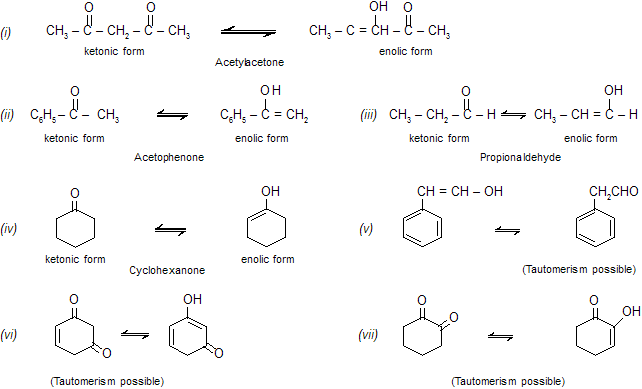
 |
Alkyl cyanides (RCN) and alkyl isocyanides (RNC) are also examples of tautomerism.
Similarly, nitro compounds also show tautomerism.
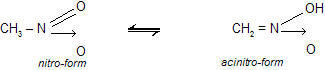
1. Tautomerism involves a change in the position of atom (generally hydrogen), while resonance involves a change in the position of the unshared or
2. Tautomers are definite compounds and may be separated and isolated. Resonating structures are only imaginary and can’t be isolated.
3. The two tautomeric forms have different structures (i.e. functional groups). The various resonating structures have the same functional group.
4. Tautomers are in dynamic equilibrium with each other, resonating structures are not in dynamic equilibrium.
5. Tautomerism has no effect on bond length, while resonance affects the bond length (single bond is shortened while the double bond becomes longer).