Isomerism-3
- Tautomerism does not lower the energy of the molecule and hence does not play any role in stabilising the molecule, while resonance decreases the energy and hence increases the stability of the molecule.
8. Tautomerism can occur in planar as well as non-planar molecules, while resonance occurs only in planar molecules.
Distinction of tautomerism from isomerism. In fact there is no sharp line of distinction between isomers and tautomers since some substances which are isomers under normal conditions can be converted into tautomeric forms under more drastic conditions. For example, propyl and iso-propyl bromides are isomeric compounds under normal conditions but form an equilibrium mixture on heating at 250°C in a sealed tube.
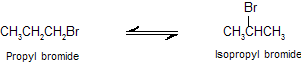
And hence dynamic isomerism is a better term for this phenomenon than tautomerism.
Distinction of tautomerism from molecular rearrangement. Although there is no sharp difference between tautomerism and molecular rearrangement, yet the two can be distinguished by the fact that the former is a rapid and reversible phenomenon whereas the latter is neither reversible nor rapid.
Stereo isomerism
When isomers have the same structural formula but differ in relative arrangement of atoms or groups in space within the molecule, these are known as stereoisomers and the phenomenon as stereoisomerism. The spatial arrangement of atoms or groups is also referred to as configuration of the molecule and thus we can say that the stereoisomers have the same structural formula but different configuration. Stereoisomerism is of two types.
(i) Geometrical isomerism
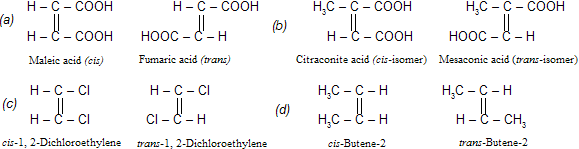
The isomers which possess the same structural formula but differ in the spatial arrangement of the groups around the double bond are known as geometrical isomers and the phenomenon is known as geometrical isomerism. This isomerism is shown by alkenes or their derivatives. When similar groups lie on the same side, it is the cis-isomer; while when the similar groups lie on opposite sides, the isomer is trans. For example,
Remember that geometrical isomerism is possible only when each of the doubly bonded carbon atom has two different groups (see examples above). Thus compounds of the following type will not show geometrical isomerism.
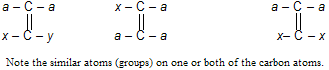
Distinction between cis -and trans- isomers. (a) Generally, the cis-isomer (e.g. maleic acid) cyclises on heating to form the corresponding anhydride while the trans-isomer does not form its anhydride at all.
(b) The cis-isomer of a symmetrical alkene (alkenes in which both the carbon atoms have similar groups) has a definite dipole moment, while the trans-isomer has zero dipole moment. For example, 1, 2-dichloroethylene and butene-2.

In trans-isomer of the symmetrical alkenes, the effect produced in one half of the molecule is cancelled by that in the other half of the molecule.
In case of unsymmetrical alkenes, the cis-isomer has higher dipole moment than the corresponding trans-isomer. For example,
The E and Z Nomenclature of Geometrical Isomers. As discussed earlier, the geometrical isomerism is possible in structures of the following three types.
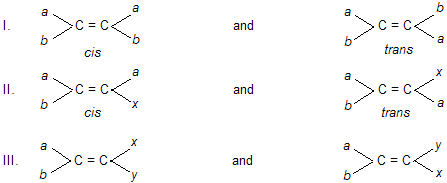
In the first two types, the geometrical isomers are labelled as cis and trans on the basis of the fact that the common groups are on the same or opposite sides of the double bond. But in type 3 where all the four substituents are different, cis-trans type of isomerism cannot be applied. Moreover, the cis-trans system (also syn-anti system in oximes) is often ambiguous because the cofigurational descriptions have not been defined according to any general and clear set of rules. So an unambiguous system of configurational assignments for all types of structures showing geometrical isomerism was developed in 1968. This system is known as E-Z system of nomenclature and is based upon the sequence rules of Cahn, Ingold and Prelog originally developed for naming optical isomers on the R-S system. The following procedure is followed in specifying the configuration of such compounds.