Isomerism-4
(i) Assign the priority order to the two groups attached to each of the doubly bonded carbon atoms in accordance with the sequence rules. Sequence rules are for determining the priority order to atoms or groups attached to doubly bonded carbon atoms.
(a) Higher priority is assigned to atoms (directly attached to the carbon atom) of higher atomic number.
(b) If isotopes of the same element are attached, the isotope with higher mass number will have a higher priority. If the priority cannot be decided by this rule, it is then determined by comparing the next atom in the group and so on.
(c) A doubly or triply bonded atom is considered equivalent to two or three such atoms. Thus a carbonyl group is considered as if carbon has two single bonds with oxygen, i.e.,
By the application of these rules some common substituents have been given the following priority sequence:-
(ii) Select the atom/group with higher priority on each doubly bonded carbon. If the atoms/groups of higher priority (denoted by 1) on each carbon are on the same side of the double bond, the isomer is assigned the configuration Z (from the German word, zusammen meaning together). On the other hand, if the atoms/groups of higher priority on each carbon are on the opposite sides of the double bond, the isomer is assigned the configuration E (from the German word entgegen meaning against).
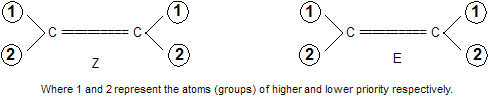
Now let us consider the example of an alkene in which one of the doubly bonded carbon atoms has Br and I and the other has F and Cl. Now since I has a higher atomic number than Br, it is assigned higher priority (1); similarly Cl is of higher priority than F on the second olefinic carbon atom. Thus the E and Z configuration of the two isomers of 1-bromc-2-chloro-2-fluoro-1-iodoethene are assigned as below.
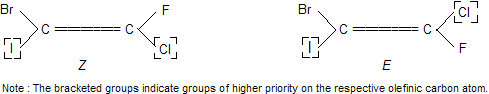
Thus the cis- and trans-isomers of 2-butene become Z-and E-2-butenes respectively.
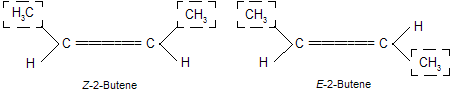
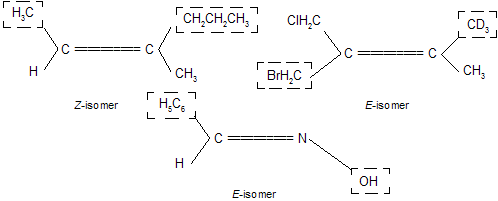
Similarly, following structures are assigned to the configuration mentioned below them.

In ketoximes the prefixes syn and anti indicate which group of ketoxime is syn (on the same side) or anti (on the opposite sides) to the OH group. For example,
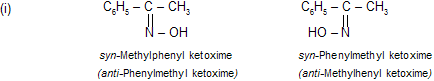
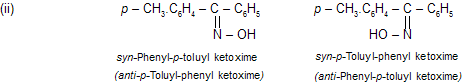 |
However, remember that all aromatic ketoximes do not show geometrical isomerism e.g., (C6H5)2C = NOH, (benzophenone oxime) having two similar aryl groups does not show geometrical isomerism.
Interconversion of Cis- Trans isomers
The cis and trans isomers of alkenes do not interconvert under ordinary conditions because of
The geometrical isomers can be interconverted if energy of more than 68 kcal/mole (the
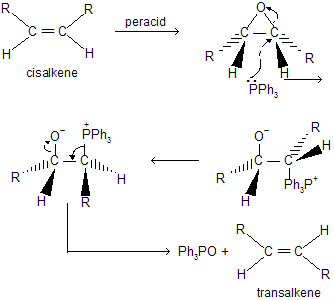
Interconversion of double bond diastereomers can also be brought Via epoxidation deoxygenation sequence. The nucleophile attack by phosphours regents example, triphenyl phosphine at the oxirane carbon leads to inversion of configuration and yields a charge separated intermediate (a betaine). This undergoes elimination Via a four center cyclic transition state which requires a 180° rotation around the C — C bond to establish the appropriate geometry. Therefore, if these are cis in the oxirane they become trans in the alkene.