Isomerism-5
Conversion of cis into trans or vice versa by heat or uv medium or by free radical initiator is known as stereomutation. In the presence of free radical double bond first gets converted into single bond then free rotation around this single bond results in inversion of configuration. Finally, regeneration of double bond occurs.
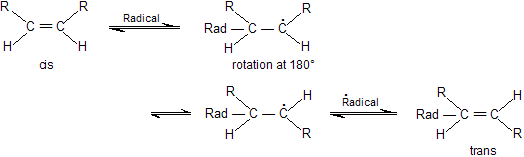
Geometrical isomerism also occurs in some saturated cyclic diols, di halide and di carboxylic acids.

(ii) Optical isomerism
This type of isomerism arises from different arrangements of atoms or groups in three dimensional space resulting in two isomers which are mirror image of each other. Optical isomers contain an asymmetric (chiral) carbon atom ( a carbon atom attached to four different atoms or groups) in their molecules. For example, lactic acid having four different groups on the central carbon atom is optically active; while succinic acid having two similar atoms on the central carbon atom is optically inactive.
Optical isomers have similar chemical and physical properties and differ only in their behaviour towards plane polarised light. The isomer which rotates the plane polarised light to left is known as laevo (l) while that which rotates the plane polarised light to the right is known as dextro (d). For example,
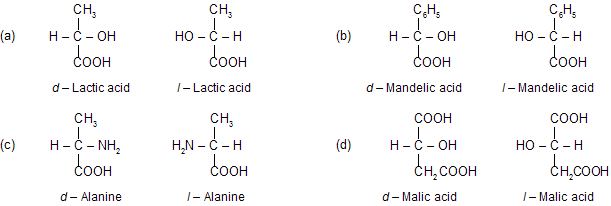
Note that the d– and l – forms of a compound are non–superimposible mirror image of each other and such pairs are known as enantiomorphs or enantiomers. A compound can exist in enantiomeric forms if it has an asymmetric carbon atom and is devoid of the elements of symmetry, viz. (i) a plane of symmetry, (ii) a centre of symmetry and (iii) an alternating axis of symmetry. If a molecule possesses any of the above elements of symmetry, it is symmetrical; on the other hand, if it does not possess either of these elements of symmetry, it is asymmetric and hence is optically active and can exist in d and l forms.
The number of optical isomers in a molecule containing n number of different asymmetric carbon atoms is given by the relation 2n. Furtermore, there will be 2n–1 pairs of enantiomer and the same number of racemic modifications. Racemic modification
The number of optical isomers in a compound containing n number of similar asymmetric carbon atoms is always less than 2n. The classical and most important example is tartaric acid, CH(OH)COOH.CH(OH)COOH which can exist in the following isomeric forms.
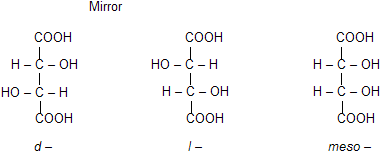
(i) d–Tartaric acid. It rotates the plane polarised light to the right. The rotation due to the upper half is strengthened by rotation due to the lower half. It has no plane of symmetry.
(ii) l –Tartaric acid. It rotates the plane polarised light to left. Here again rotation due to upper half is strengthened by rotation due to lower half. It also has no plane of symmetry. The d – and l – tartaric acids are mirror–image of each other (enantiomers).
(iii) r – Tartaric acid. It is equimolecular mixture of the d – and l – forms and hence optically inactive (eg. r – lactic acid) due to external compensation.
(iv) m – Tartaric acid. It possesses a plane of symmetry (denoted by dotted line) and hence superimposes on its mirror image (i.e., they are identical) and hence it is optically inactive. The optical inactivity is said to be due to internal compensation as the rotation due to the upper half of the molecule is balanced by the equal but opposite rotation due to the lower half. The meso–isomer cannot be resolved into active (d – and l –) isomers (difference from racemic tartaric acid).