Isomerism-6
Remember that stereoisomers which are not mirror image (enantiomers) are known as diastereomers or diastereoisomers. Thus m – tartaric acid constitutes the diastereomer of d – as well as of l – tartaric acid.
Prediction of number of optical isomers
(i) When the molecule is unsymmetrical
Number of d and l isomers (a) = 2n (active) Where n is the number of chiral carbon atom (s). Common example is CH3.CHBr.COOH 21 = 2 |
(ii) When the molecule is symmetrical and has even number of chiral carbon atoms
| Number of d and l isomers (a) = 2(n–1) Number of meso forms (m) = 2(n/2 –1) Common example is tartaric acid, HOOC. CHOH. CHOH.COOH |
(iii) When the molecule is symmetrical and has an odd number of chiral carbon atoms.
| Number of d and l forms (a) = 2(n–1) –2(n/2 – ½) Number of meso forms (m) = 2(n/2 – ½) |
Optical Isomerism in compounds containing no chiral carbon atom
As described earlier that the basic requirement for a compound to be optically active is its non–superimposibility of its mirror image. Although the largest number of known optically active compounds are optically active due to the presence of chiral carbon atom, some compounds are also known which do not possess any chiral carbon atom, but on the whole their molecules are chiral (such molecules were earlier called dissymmetric); hence they are optically active. Various types of compounds belonging to this group are allenes, alkylidene cycloalkanes, spiro compounds (spiranes) and properly substituted biphenyls.
Allenes. Allenes are the organic compounds of the following general formulae.
Allenes exhibit optical isomerism provided the two groups attached to each terminal carbon atom are different, i.e.
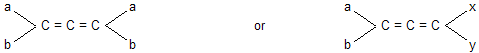
Alkylidenes cycloalkanes and spiro compounds. When one or both of the double bonds in allenes are replaced by one and two rings, the resulting systems are respectively known as alkylidene cycloalkanes and spiranes.
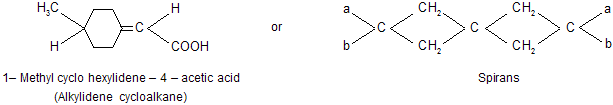
Biphenyls. Suitably substituted diphenyl compounds are also devoid of individual chiral carbon atom, but the molecules are chiral due to restricted rotation around the single bond between the two benzene nuclei and hence they must exist as two non–superimposible mirror images of each other. Such type of stereoisomerism which is due to restricted rotation about single bond, is known as atropisomerism and the stereoisomers are known as atropisomers. Examples,

Racemic Mixture or Racemic Modification
As described earlier, a racemic modification is an equimolecular mixture of a pair of enantiomers, i.e., (+) – and (–) – forms and is denoted by ![]() Racemic mixture is generally obtained in the following two ways.
Racemic mixture is generally obtained in the following two ways.
(i) By mixing equal amounts of the two enantiomers.
(ii) By synthesis. The synthesis of a chiral compound from achiral compound in the absence of optically active agent or circularly polarised light always produces a racemic modification. For example, the formation of lactonitrile from acetaldehyde always results in a racemic modification in the following manner:-
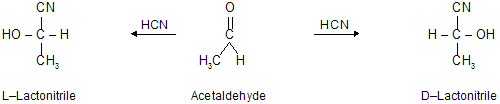
Resolution
Separation of dl–mixture of a compound into d and l isomers is known as resolution. This can be done by several methods, viz. mechanical, biochemical and chemical method. Chemical method involves the formation of diastereomers and is found to be the best method for resolution.
Walden inversion (Optical inversion). The conversion of d–form of an optically active compound into l–form of the same or of different compound or vice–versa is known as Walden inversion or optical inversion (P. Walden in 1895). For example, d – malic acid when treated with PCl5 gives l – chlorosuccinic acid, i.e., inversion in configuration has taken place. The l –chlorosuccinic acid may also be converted back to malic acid with or without change in configuration which actually depends upon the nature of the reagent.
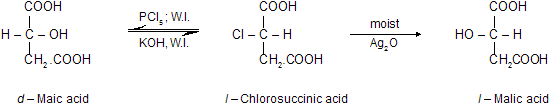
Thus among other factors, nature of the reagent plays an important role in Walden inversion. It has been observed that mild or weak reagent like Ag2O do not cause Walden inversion while strong reagents like KOH and PCl5 cause Walden inversion.
Remember that Walden inversion follows SN2 mechanism which involves the inversion of configuration while SN1 mechanism involves racemisation.