METALLURGY - 1
2. METALLURGY
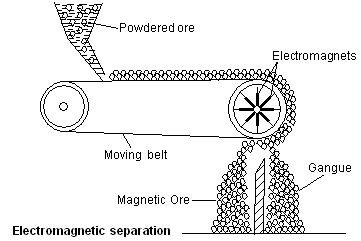
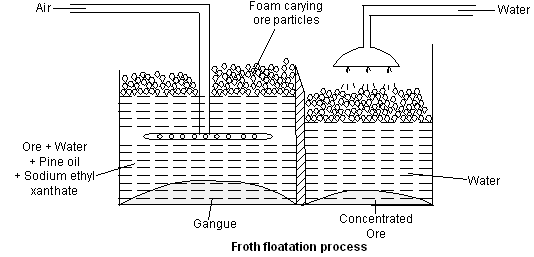
Chemical methods
- Calcination : It involves heating of the ore below its fusion temperature in absence of air. This step expels organic matter and moisture from the ores. It can remove moisture from hydrated oxides or carbon dioxide from carbonates. For example :Al2O3·2H2O
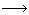 Al2O3 + 2H2O
Al2O3 + 2H2O
2Fe2O3·3H2O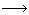 2Fe2O3 + 6H2O
2Fe2O3 + 6H2O
CuCO3·Cu(OH)2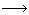 2CuO + CO2 + H2O
2CuO + CO2 + H2O
Calcination makes the ore porous. The step is generally performed in reverberatory furnace. - Roasting : It is also heating of the ore either alone or with some other material usually in presence of air below its fusion temperature. roasting is generally done in a reverberatory furnace or in a blast furnace. In roasting definite chemical changes like oxidation, chlorination, etc., take place while in calcination these occurs only expulsion of organic matter, water, carbon dioxide etc., i.e., it does not involve any major chemical change.
The roasting process may be any one of the following types :- Oxidising roasting : This is very common type of roasting in metallurgy and is carried out to remove sulphur and arsenic in the form of their volatile oxides such as SO2 and As2O3 respectively. The ores are simultaneously converted into corresponding oxides. This type of roasting is generally applied in ores of lead, zinc, nickel, copper, etc.S + O2
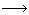 SO2
SO2
4As + 3O2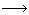 2As2O3
2As2O3
2PbS + 3O2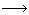 2PbO + 2SO2
2PbO + 2SO2
2CuS + 3O2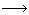 2Cu2O + 2SO2
2Cu2O + 2SO2 - Partial oxidising or sulphating roasting : This type of roasting is carried out at a temperature below the melting point of the charge and air is admitted. Part of the sulphide ore is oxidised to sulphate and the rest is converted into oxide. For example, the roasting of galena leads to the formation of a mixture of lead oxide and lead sulphate.
2PbS + 3O2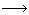 2PbO + 2SO2
2PbO + 2SO2
PbS + 2O2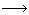 PbSO4
PbSO4 - Chlorinating roasting : This type of roasting is done in the case of silver ore. The ore, argentite is mixed with common salt and the mixture is heated in the presence of air. The final product is the chloride of the metal.
Ag2S + 2NaCl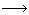 2AgCl + Na2S
2AgCl + Na2S
- Oxidising roasting : This is very common type of roasting in metallurgy and is carried out to remove sulphur and arsenic in the form of their volatile oxides such as SO2 and As2O3 respectively. The ores are simultaneously converted into corresponding oxides. This type of roasting is generally applied in ores of lead, zinc, nickel, copper, etc.