METALLURGY - 3
(c) Electrolytic reduction : The oxides of the highly electropositive metals like Na, K, Mg, Ca, Al, etc., cannot be reduced easily with carbon at moderate temperatures. For reduction, a very high temperature is required at which the metal may combine with carbon to form a carbide. These metals are thus extracted by the electrolysis of their oxides, hydroxides or chlorides in fused state. Sometimes, a small amount of some other salt is added as to lower the fusion temperature or to increase the conductivity or both. The metal is liberated at the cathode. Sodium is obtained by the electrolysis of fused mixture of NaCl and CaCl2 (Down's process) or by electrolysis of fused sodium hydroxide (Castner's process).
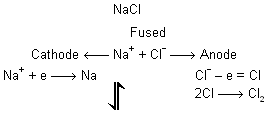
(d) Hydrometallurgy : This process is based on the fact that more electropositive metal can displace less electropositive metal from its salt solution. The ore is teated with such chemical reagents which convert it into soluble compound. By the addition of more electropositive metal to the filtrate, the metal present in the ore can be precipitated. The following two examples illustrate this process.
(i) Extraction of copper : Malachite ore is first roasted.
CuCO3·Cu(OH)2 ![]() 2CuO + H2O + CO2
2CuO + H2O + CO2
Copper oxide obtained is dissolved in sulphuric acid.
CuO + H2SO4 | ||||
| (Soluble) | ||||
To the solution of copper sulphate, scrap iron is added which precipitates copper.
CuSO4 + Fe | ||||
| ppt. Soluble | ||||
(ii) Extraction of silver : The ore is dissolved in sodium cyanide solution.
2NaAg(CN)2 + Zn | ||||
| Soluble | ppt. | |||
(e) Amalgamation process : This method is used for the extraction of noble metals like gold, silver, etc., from the native ores. The finely powdered ore is brought in contact with mercury which combines with the particles of the metal present in the ore and form amalgam. The metal is recovered from the amalgam by subjecting it to distillation, where the mercury distills over leaving behind the metal.
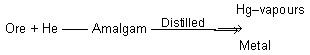
3. Third operation-Refining or purification : The metals obtained by the application of above reduction methods from the concentrated ores are usually impure. These impure metals may be associated with small amounts of (a) unchanged ore, (b) other metals produced by the simultaneous reduction of their compounds originally present in the ore, (c) non-metals like silicon, carbon, phosphorus, etc. (d) residual slag, flux, etc. The impure metal is thus subjected to some purifying processes known as refining in order to remove the undesired impurities. The following refining processes may be applied depending upon the nature of the metal under treatment and the nature of the impurities.
(a) Liquation process : This process is based on the difference in fusibility of the metal and impurities. When the impurities are less fusible than the metal itself, this process is employed. The metal melts and flows down leaving behind the impurities on the hearth. This method is used to purify the metals like Bi, Sn, Pb, Hg, etc.
(b) Distillation : This process is used for those metals which are easily volatile. The impure metal is heated in a retort and its vapours are separately condensed in a receiver. The non-volatile impurities are left-behind in the retort. This is used for the purification of Zn, Cd, Hg, etc.
(c) Pyrometallurgical oxidation process : This process is used when the impurities have a rgeater affinity for oxygen than the metal itself. This method is usually employed for refining the metals like Fe, Cu, Ag, etc. The oxidation is done by various ways.
(i) Cupellation : The impure metal is heated in a cupel or oval shaped crucible made of bone ash or cement and a blast of air is passed over the molten mass. The impurities get oxidised and removed with the blast of air. For example the impurity of lead present in silver is removed by cupellation process.
(ii) Bessemerisation : The impure-metal is heated in a furnace and a blast of compressed air is blown through the molten mass. The impurities get oxidised. For example, the molten pig iron is taken in a bassemer converter and compressed air is passed which oxidises the impurities.
2Mn + O2 2C + O2 | ||
| Slag | ||
(iii) Poling : The impure metal containing oxides as impurity can be purified by this method. The molten impure metal is stirred with green poles of wood. The green poles of wood release the hydrocarbon gases which reduce the oxide impurities. This method is especially used in the purification of copper. (old method)
(d) Electrolytic refining of metals : Many of the metals such as copper, silver, gold, aluminium, lead, etc., are purified by this method. This is perhaps the most important method. The impure metal is made anode while a thin sheet of pure metal acts as a cathode. The electrolytic solution consists of generally an aqueous solution of a salt or a complex of the metal. On passing the current, the pure metal is deposited on the cathode and equivalent amount of the metal gets dissolved from the anode. Thus, the metal is transferred from anode to cathode through solution. The soluble impurities pass into the solution while the insoluble one, especially less electropositive impurities collect below the anode as anodic mud or anode sludge,. Some examples are given below :
(i) Purification of copper
| Impure metal—Anode; | Thin sheets of copper—Cathode |
| Electrolyte—An aqueous solution of copper sulphate containing H2SO4. | |
A current of 1.3 volt is used. Anodic mud contains Ag, Au, Pt, Pd, etc., and impurities like Fe, Zn, Ni, etc., pass into the solution. 99.9% pure copper is obtained.
(ii) Purification of lead
| Impure metal—Anode; | Thin sheets of pure lead—Cathode |
| Electrolyte—A solution of lead silico fluoride PbSiF6 containing 8 – 10% of H2<>/subSiF6 | |
(e) Special methods
(i) Mond’s process : Nickel is purified by this method. Impure nickel is treated with carbon monoxide at 60–80°C when volatile compound, nickel carbonyl, is formed. Nickel carbonyl decomposes at 180°C to form pure nickel and carbon monoxide which can again be used.

(ii) Van-Arkel process : This methods is generally applied for obtaining ultrapure metals. The impure metal is converted into a volatile compound while the impurities are not affected. The volatile compound is then decomposed electrically to get the pure metal.
Ti, Zr, Hf, Si, etc., have been refined by this method. The method is quite expensive.
