Nucleophilic-Substitution-2
(i) Features of SN2 Reaction :
- Occurs mainly in 1o alkyl halide due to small size and little stericfactor.
- Order of reaction is 2 but total step in the mechanism is one.
- Speed of the reaction increases by nonprotic solvent example DMF and DMSO.
- Back attack of the nucleophile is there so that stereo chemical inversion occurs.
- Elemental effect so that RI is maximum reactive.
- no carbocation, so no molecular rearrangement. Only transition state is formed.
(ii) Stereochemistry of SN2 reaction
Two stereochemical possibilities are present here. In the pathway the nucleophile simply assumes the position occupied by the leaving group. It attacks the reactant at the same face (front side) from which the leaving group departs. This is called “front-attack displacement,” or substitution with retention of configuration.
Other option is attack of nucleophile from back side of the molecule where no heterolytic fission occurs, actually transtion state is formed. From these two opposite stereochemical possibilities, the correct one was determined by carrying out nucleophilic substitution reactions with optically active alkyl halides. If we determine by experiment that hydrolysis of

2-chloro octane in the presence of hydroxide ion gave 2-octanol having a configuration opposite to that of the starting alkyl halide. (back attack)

Nucleophile substitution in this above case occurred with inversion of configuration by back attack of nucleophile consistent with the following transition state representation :
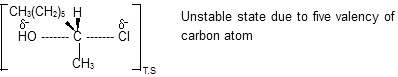
Substitution reaction that proceed the SN2 mechanism are stereospecific in nature and proceed with inversion of configuration at the carbon that bears the leaving group. There is a stereoelectronic requirement for the nucleophile to approach carbon from the side opposite the bond to the leaving group. Organic chemists often speak of this as a Walden inversion. For such reaction, rate of chemical reaction depends upon concentration of alkyl halide and nucleophile both so order of reaction is two with single step process.