Nucleophilic-Substitution-3
Mechanism unimolecular (SN1) nucleophilic substitution
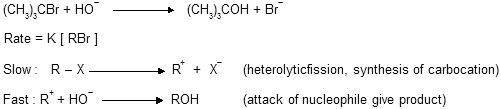
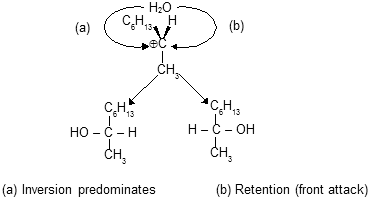
Now nucleophilic attack can takes place at any time after the heterolysis of slow step and thus can involve any species from the initially formed ion pair to the free carbocation, attack on the free carbocation is random and yields the racemic modification. Actually the anion surrounds the planar carbocation and thus shields this side from front attack ; as a result, back side attack is preferred to the extent. Front side attack occurs before the ion pair has completely separated, inversion of configuration competes with racemization.
(i) Features of SN1 Reaction
1. Rate of reaction = K [RX], order of reaction is one which depends only upon alkylhalide concentration but it is a two step process.
2. Carbocation formation takes place so that molecular rearrangement may occur.
3. Racemization takes place by both back side and front side attack.
4. Chemical reactivity 3o > 2o > 1o becasue ter. alkyl halide forms maximum stable carbocation.
5. Elemental effect, so that reactivity of differant halide is RI > RBr > RCl >> RF