Nucleophilic-Substitution-4
(ii) Mechanism and stereochemistry
Tertiary alkyl halides are practically inert to substitution by the SN2 mechanism because of hindrance, they forms highly stable carbocation so undergo substition by heterolytic fission.
The hydrolysis of tert-butyl chloride, a first-order rate law known as SN1 reaction.
They found that the rate of hydrolysis depends only on the concentration of tert-butyl chloride. Adding the stronger nucleophile hydroxide ion, moreover, causes no change on rate of reaction.
The overall reaction :

Step A: The alkyl halide break down into a carbocation and a halide ion.
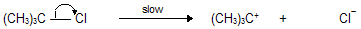
Step B: The carbocation formed in step 1, rapidly reacts with a water molecule (lewis base). Water is a nucleophile. This step completes the nucleophilic substitution stage of the mechanism and yields an oxonium ion (acid base complex).

Step C : This step is a fast acid-base reaction with nucleophilic substitution. Water acts as a base to remove a proton from the oxonium ion to give the final product of the reaction.

The proposed mechanism is called SN1, standing for substitution nucleophilic unimolecular. The first step, a unimolecular dissociation of the alkyl halide to form a carbocation as the key intermediate, is rate -determining step.
Effect of solvent on the rate of nucleophilic substitution
To understand effect of the solvent at the rate of nucleophilic substitution reaction, following points must be considered.
1. Properties of the solvent which are responsible to effect the rate.
2. How does the activation energy of the rate-determining step respond to this property of the solvent?
(i) Effects of solvent on the SN1 reaction
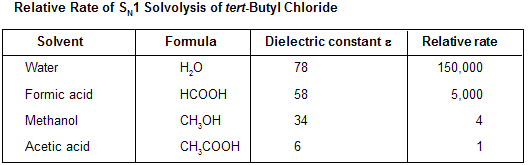
Solvent effect on SN1 reaction is mainly based on its polarity and polarity of solvent is measured by its dielectric constant. Ability of solvent to attract solute particles and minimize the force of attraction between them. The standard dielectric is a vacuum, which has a value of e of exactly 1. Higher the value of dielectric constant, higher the ability to support separation of charged ions so solvents with high DEC are known as polar solvents. As the value of DEC increases the rate of solvolysis also increases.
According to the SN1 mechanism, a neutral alkyl halide molecule ionizes to a positively charged carbocation and a negatively charged halide ion in the rate-determining step. As the alkyl halide approaches the transition state for this step, a partial positive charge develops on carbon and a partial negative charge on the halogen. Polar and nonpolar solvents are similar in their interaction with the starting alkyl halide, but differ markedly in how they affect the energy of the transition state. A solvent with a low dielectric constant has little effect on the energy of the transition state, while one with a high dielectric constant stabilizes the charge - separated transition state, lowers the activation energy, and increase the rate of reaction.