Nucleophilic-Substitution-5
(ii) Effects of solvent on the SN2 Mechanism
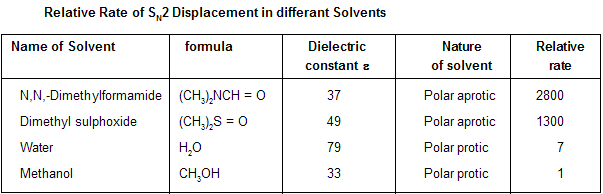
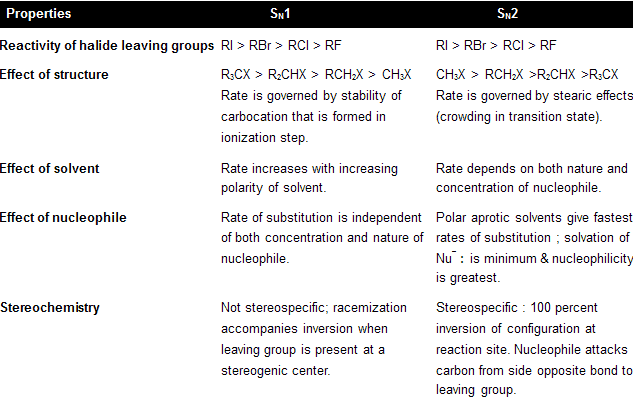
Solvent on SN2 reactions are required in polar form because ionic substances, such as sodium, potassium, Rubidium salts are not fully soluble in nonpolar solvents, so they cannot give enough amount of nucleophile to allow the reaction to occur at rapid speed. But remember, effect of solvent polarity on the rate of bimolecular substitution is small. Primary factor that effect the rate of SN2 reactions is whether or not the polar solvent is protic or aprotic.
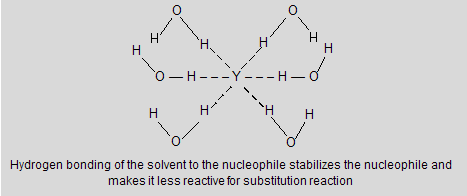
In polar protic solvents like HOH, ROH and RCOOH they all have –OH groups that allow them to form hydrogen bonding with anionic nucleophiles. Solvation forces such as these stabilize the anion and suppress its nucleophilicity. Aprotic solvent does not contain –OH group so they do not involve in solvation of anion, leaving them nekdly to express their better nucleophilic character. The second order kinetics constants of 1-chloropropane by azide ion in polar protic solvent is very low for example in methanol and water.

The large rate enhancements observed for SN2 in polar aprotic solvents. An example can be seen in the preparation of alkyl
cyanides (nitriles) by the reaction of potassium cyanide with alkyl halides :

When the reaction was carried our in aqueous methanol as the solvent, pentyl bromide was converted to pentyl cyanide in 70 percent yield by heating with sodium cyanide. While this is a perfectly acceptable synthetic reaction, a period of over 20 hours was required. Changing the solvent to dimethyl sulfoxide brought about an increase in the reaction rate sufficient to allow the less reactive substrate pentyl chloride to be used instead, and the reaction was complete (90 percent yield) in only few minutes.