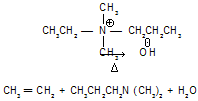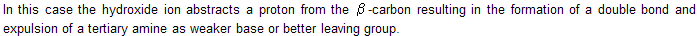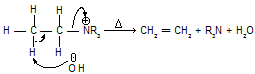Nucleophilic-Substitution-8
R2C == CR2 > RCH == CR2 > RCH == CHR > RCH == CH2 > CH2 == CH2
The greater stability of the more substituted alkene is sometimes explained on the basis of hyperconjugation, more the number of hyperconjugative structure, more is the stability of alkene.
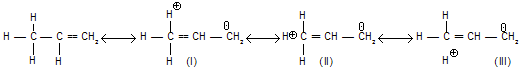

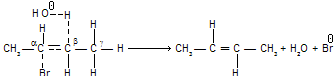
b. The Hoffmann Rule
It states that charged substrates (quaternary ammonium and sulphonium salts) yield predominantly the least substituted alkene.
Let us first examine the mechanism of the elimination reaction of a quaternary ammonium hydroxide. On heating, these compounds yield an alkene and a tertiary base.