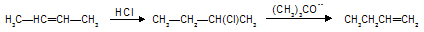Nucleophilic-Substitution-9
It has been observed that quaternary ammonium hydroxides as well as sulphonium salts preferably yield less substituted alkenes.
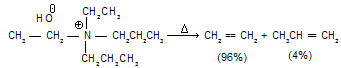

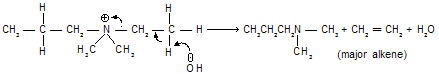
For the same reason an isobutyl group shows still less tendency to lose a proton if either an n-propyl or an ethyl group is available in the molecule.
Stearic factors also play a big role in Hoffmann eliminations. It is apparent that the groups eliminated in these reactions are bulkier than those lost in the Saytzeff type of eliminations. So that, it has been found that E2 reactions of alkyl halides can be forced to follow Hofmann type of elimination by sufficiently increasing the bulk of the attacking base or by increasing the degree of branching in the substrate. Treatment of following bromoderivative with base, for instance, gives 1-alkene in preference to 2-alkenes mainly due to stearic strain resulting from the interaction of methyl and tert-butyl groups in the latter alkene.
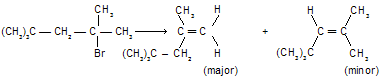
A similar stearic effect seems to operate when the size of the attacking base is increased, for example
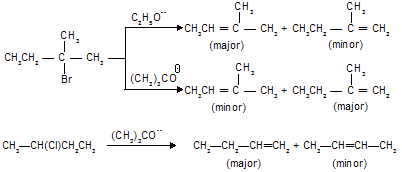
This reaction is used for conversion of nonterminal into terminal derivatives for example