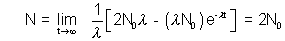Nucleus - 6
(2) A small Quantity of solution containing Na-24 radioactive nuclei
t1/2 = 15h of activity 1.0 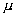 ci is injected into the blood of a person. A sample of blood of volume 1cm3 taken after 5th, throws an activiy of 296 d/ min.
ci is injected into the blood of a person. A sample of blood of volume 1cm3 taken after 5th, throws an activiy of 296 d/ min.
Determine the total voume of blood in the body of person
Let volume of blood be V
activity of 1 cm3 initially = 
activity after 5 hrs = A1 = 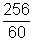 = 4.93 dps
= 4.93 dps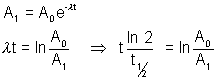
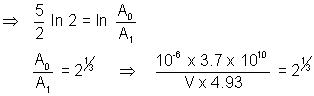
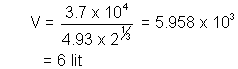
(3) 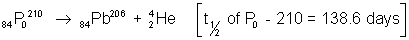 Polinum is used forgeneration of electricity, assuming the efficiency of the electricity, assuming the efficiency of the electric machine to be 10% how much P0 is required 693 days. Also find the intial activity of material.
Polinum is used forgeneration of electricity, assuming the efficiency of the electricity, assuming the efficiency of the electric machine to be 10% how much P0 is required 693 days. Also find the intial activity of material.
(mass of nucleii P0 - 210 = 209.98264 u Ph - 206 = 205.97440 u 2He4 = 4.00260 u)
mass defect = (209.98264) - (205.97440 + 4.00260) = 000564u = 5.64 x 10-3 x 931Mev = 8.34 x 10-3J.
No. of fissions required for 1.2 x 107J = 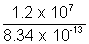 = 1.43 x 1019
= 1.43 x 1019
Since efficiency of machine is 10% Actually no. of fissions occuring = 1.43 x 1019 x 10 = 1.43 x 1020
Actually no. of fissions occuring = 1.43 x 1019 x 10 = 1.43 x 1020
mass of polinium required = 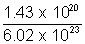 x 210 = 0.0485g
x 210 = 0.0485g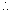 at the end of 693days 0.0483g of P0 must be present
at the end of 693days 0.0483g of P0 must be present
693 days = 138.6 x 5 = 5 x t1/2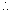 intial mass of polinium = 0.0483 x 25 = 0.0483 x 32 = 1.5456g
intial mass of polinium = 0.0483 x 25 = 0.0483 x 32 = 1.5456g
intial activity =…N0=…..X 1.43 x 1020 x 32
= 2.2 x 1019/day
Hard:
- Nuclei of a radio active element A are being produced at a constant rate
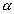 . The element has a decay constant
. The element has a decay constant 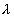 . At time t = 0, there are N0 nuclei of the element.
. At time t = 0, there are N0 nuclei of the element.
(a) Calculate the number No of Nuclei of A at time t.
(b) If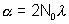 after half life of a , and also the limiting value of Nas t
after half life of a , and also the limiting value of Nas t 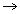
Solution:- Rate of production =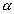
rate of decay =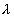 [n]
[n]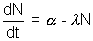 integrating
integrating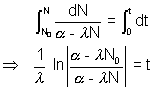
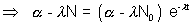
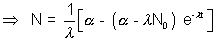 (Ans to (a) part)
(Ans to (a) part)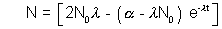
at t =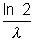
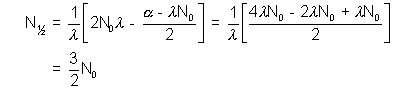
limiting value i.e. as t