organometallic-grignard-reagent-4
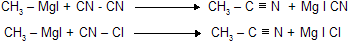
(x) Reaction with Cyanogen and Cyanogen chloride
Alkyl cyanides are produced when Grignard reagents react with cyanogen and cyanogen chloride .
(xi) Reaction with Iodine
When an alkyl magnesium chloride or bromide is treated with iodine, alkyl iodides are formed.
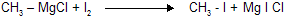
A good method for preparing an alkyl iodide from the corresponding alkyl chloride or bromide.
(xii) Reaction with Inorganic halides
Organometallic and organo - nonmetallic compounds result when Grignard reagents react with inorganic halides. For example :

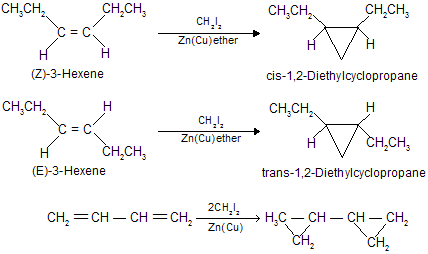
Synthesis of cyclopropane by an organic reagent
Zinc reacts with alkyl halides in a manner similar to that of magnesium.
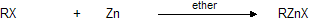
Organozinc reagents are not nearly so reactive toward aldehydes and ketones as Grignard reagents and organolithium compounds but are intermediates in a number of reactions with alkyl halides and dihalides.
An important organozinc compound in organic synthesis is iodomethylzinc iodide (ICH2ZnI), prepared by the reaction of zinc-copper couple [Zn(Cu), zinc that has had its surface activated with little copper] with di- iodomethane in ether.
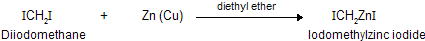
What makes iodomethylzinc iodide such a useful reagent is that it reacts with alkenes to give cyclopropanes.
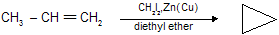
This reaction is called the Simmons-Smith reaction and mechanistically, the Simmons-Smith reaction seems to proceed by a single-step cycloaddition of a methylene (CH2) unit from iodomethylzinc iodide to the alkene :
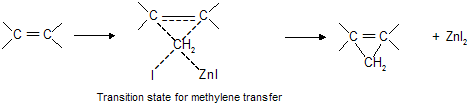
Methylene transfer from iodomthylzinc iodide is stereospecific, reacting species which are cis in the alkene remain cis in the cyclopropane also. For example