organometallic-grignard-reagent-5
Carbenes and carbenoids
Iodomethylzinc iodide is often referred to as carbenoid, because it resembles a carbene in its chemical reactions. Carbenes are neutral molecules in which one of the carbon atom has six valence electrons. Such carbons are divalent; they are directly bonded to only two other atoms and have no multiple bonds. Iodomethylzinc iodide reacts as if it was a source of the carbene H –– C –– H.
It is clear that free [:CH2] is not involved in the Simmons – Smith reaction, but there are many evidence which indicate that carbenes are formed as reactive intermediates in certain other reactions that convert alkenes to cyclopropanes. The most studied examples of these reactions involve dichlorocarbene (:CCl2) and dibromocarbene (:CBr2)
Carbenes are too reactive to be isolated and stored, so these are trapped in frozen argon for spectroscopic study at very low temperatures.
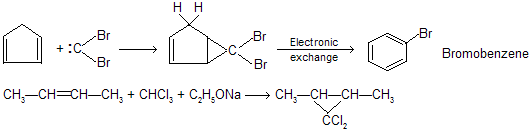
Dihalocarbenes are formed when tri halomethanes are treated with a strong base, such as potassium tert-butoxide. The tri halomethyl anion produced on proton abstraction dissociates into di halocarbene and a halide anion.
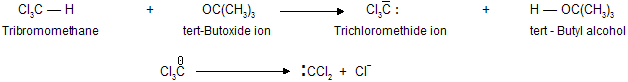
When generated in the presence of an alkene, dihalocarbenes undergo cycloaddition to the double bond to give di halo cyclopropanes :
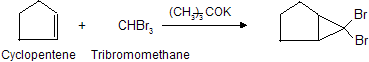
The reaction of dihalocarbenes with alkenes is stereospecific, where syn addition is observed. The process in which a di halocarbene is formed from a tri halo methane corresponds to an elimination in which a proton and a halide are lost from the same carbon. It is an example of proceeding via the organometallic intermediate K+[:CX3]– while most of elimination are included in
proceeding via the organometallic intermediate K+[:CX3]– while most of elimination are included in  for example dehydration of alcohol.
for example dehydration of alcohol.
There is a big difference of a reaction when alkenes reacts with haloform in alcoholic bases or weak bases for example
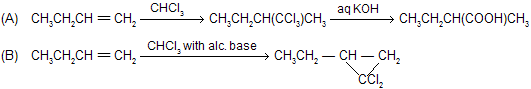
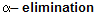 where synthesis of dichlorocarbene takes place.
where synthesis of dichlorocarbene takes place.