Stereochemistry-1 - stereogenic center
Stereo Chemistry
Chapter
Molecules that are attached to four different groups a, b, d and e are chiral.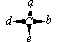
A tetrahedral carbon that is attached to four different group is known as a chiral centre, a chiral carbon atom, an asymmetric centre or an asymmetric carbon atom. Now a days a more modern term stereogenic centre is used.
It we have to find, the presence of a stereogenic centre in a given molecule then we have to check if it is chiral. For example C-2 is stereogenic centre in 2-butanol.
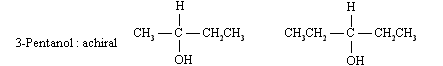
A stereogenic centre has four different groups. (a) In 2-chloropentane, C-2 satisfies this requirement. (b) None of the carbons in 3-bromopentane have four different substituents, and so none of its atoms are stereogenic centres.
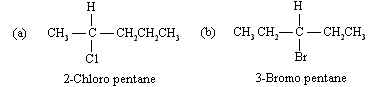
In the following examples, the stereogenic carbon is indicated by an asterisk. But remember carbons that are part of a double bond or a triple bond cannot be stereogenic centres.
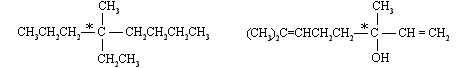
In a ring, carbon atom can be stereogenic center if it is attached to two different substituents and the path traced around the ring from that carbon in one direction is different from that traced on the other side. For example the carbon atom which is attached to methyl group in 1,2-epoxypropane, is a stereogenic centre. The sequence of groups in CH2–O as one proceeds clockwise around the ring from that carbon, but it is O–CH2 in the anticlockwise direction. In limnolesne C–4 is stereogenic centre.

Prob. Identify the stereogenic centers, if any, in
(a) 2-Cyclopenten-1-ol
(b) 3-Cyclopenten-1-ol
Answer (a) The hydroxyl-bearing carbon in 2-cyclopentene –1– ol is a stereogenic center.
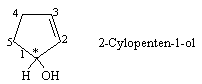
(b) There is no stereogenic center in 3-Cylcopenten-1-ol, because the sequence of atoms 1®2®3®4®5 is equivalent regardless of whether one going through clockwise or anticlockwise.
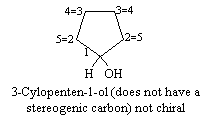
Molecules which have more than one stereogenic centre may or may not be chiral; these will be discussed later.
An optical isomer can exist in four forms :
(i) Dextrorotatory (d or +form) It rotates the plane polarised light towards clockwise directions
(ii) Laevorotatory (l or – form) It rotates the plane of polarised light towards anti clockwise.
(iii) Racemic mixture (dl or ± form) It is equimolar mixture of d and l form. It is optically inactive due to external compensation. i.e., the rotation caused by ‘d form’ is neutralised by ‘l form’
(iv) Mesoform : Optical isomer with a plane of symmetry is called meso form. It is optically inactive due to internal compensation, i.e., the rotation caused upper half of the molecule is neutralised by lower half.
Meso tartaric acid

In any molecule cause of optical isomerism can be discussed in two ways :
(i) Optical isomerism due to chirality
(ii) Optical isomerism without chirality
(i) Due to chirality : A compound is optically active if it is chiral or asymmetric, i.e., it should be non–superimposible upon its mirror image. The chirality can be achieved either due to presence of chirality carbon atom or absence of elements of symmetry.
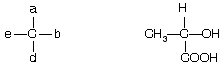
Certain structural properties related to molecular symmetry can sometimes help us determine by inspection whether a molecule is chiral or achiral. For example, a molecule that has a plane of symmetry or a centre of symmetry is superimposible on its mirror image so it is achiral.
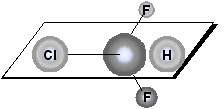
A plane of symmetry bisects a molecule so that one half of the molecule is the mirror image of the other half. The achiral molecule chlorodifluoromethane, for example, has the plane of symmetry.
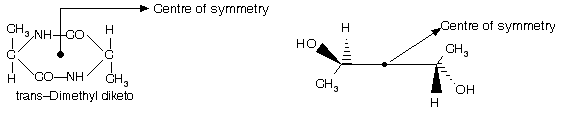
A point in a molecule is a centre of symmetry if any line drawn from it to some element of the structure will, when extended an equal distance in the opposite direction, encounter an identical element. For example cyclobutane derivative lacks a plane of symmetry, yet is achiral because it possesses a centre of symmetry.
Elements of symmetry : There are two elements of symmetry
(a) Plane of symmetry :
It may be defined as a plane which divides a molecule in two equal parts that are related to each other as an object and mirror image.
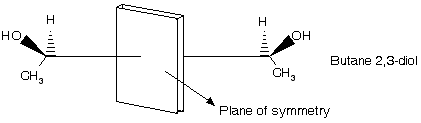
(b) Centre of symmetry : It may be defined as a point in the molecule through which if a line is drawn in one direction and extended to equal distance in opposite direction, it meets another similar group or atom.
Chiral molecules with two stereogenic centres
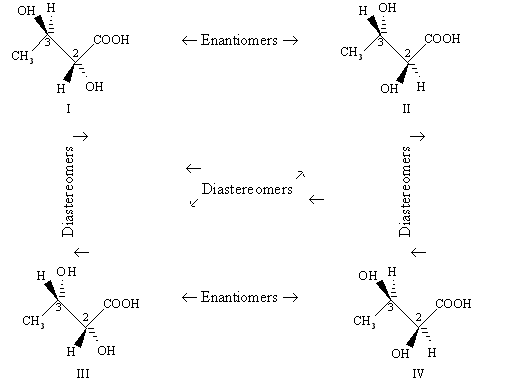
When a molecule contains two stereogenic centres, as does 2,3-dihydroxybutanoic acid, or tartaric acid type molecule how many stereoisomers are possible ?

The answer can be determined to be 4 by applying a common sense approach but remember if molecule like tartaric acid is there than mesoform becomes inactive due to molecular symmetry.
Stereoisomer I is not a mirror image of III or IC, and so it is not an enantiomer of either one. Stereoisomers that are not related as an object and its mirror image are called diastereomers; diastereomers are stereoisomers that are not enantiomers. Thus stereoisomer of III and a diastereomer of IV. Similarly, II is a diastereomer of III and IV.
In order to convert a molecule with two stereogenic centers to its enantiomer, the configuration at both centres must be changed. Reversing the configuration at only one stereogenic centre converts it to a diastereomeric structure.
When the carbon chain is vertical and like groups are on the same side of the Fischer projection, the molecule is known as the erythro diastereomer. When like groups are on opposite sides of the Fischer projection, the molecule is termed as the threo diastereomer. Thus, as seen in the Fischer projections of the stereoisomeric 2,3-dihydroxybutanoic acids, compounds I and II are erythro stereoisomers and III and IV are threo.
Keywords: groups attached, chiral center, atoms or groups, atom bearing, stereocenter are chiral, atom in a molecule, molecular formula, left handed, meso compound, quaternary ammonium