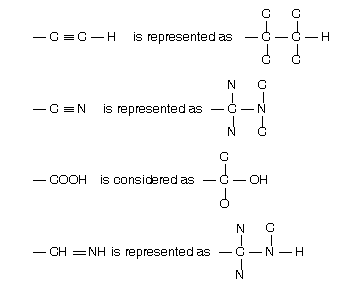Stereochemistry-2
Because diastereomers are not mirror images of each other, they can have, and often do have, markedly different physical and chemical properties. For example, the (2R,3S) stereoisomer of 3-amino-2-butanol is a liquid. While the (2S,3R) diastereomer is a crystalline solid.

Mainly four types of presentation are used to express three dimensional structure of molecules in stereo chemistry.
(1) Wedge representation
Three types of lines are used to represent the spatial arrangement of a system.
(a) Thick lines – Shows such bonds which comes towards viewer.
(b) Dotted lines – Shows such bonds which move away from the viewer.
(c) Normal lines – shows such bonds which are present in the plane of the paper
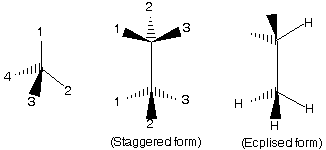
(2) Sawhorse Representation
In this representation a tilted line shows the central C — C bond.
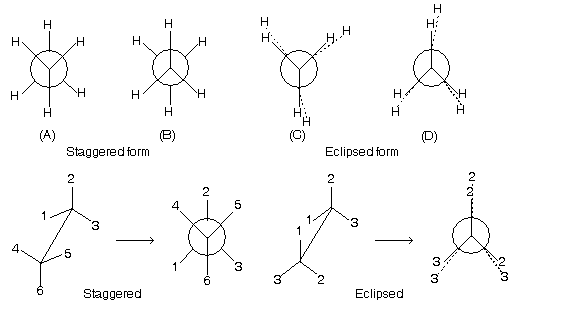
(3) Newman projection representation
Two central carbon atoms which are shown by point and circle.
(4) Fischer projection representation
Four bonds of a carbon atom are shown by the sign of +. In this presentation vertical line always shows such bonds which are away from the observer while horizontal line shows those bonds which are projected towards the observer. For example
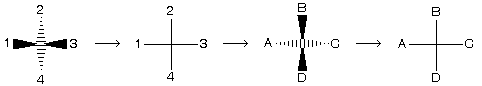 |
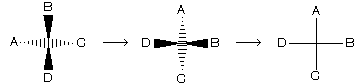 |
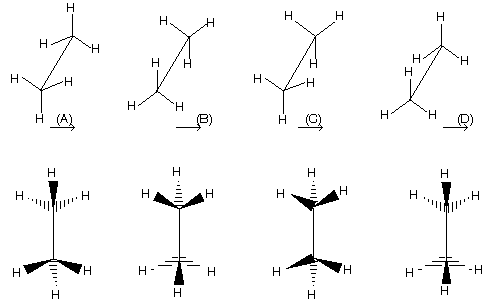
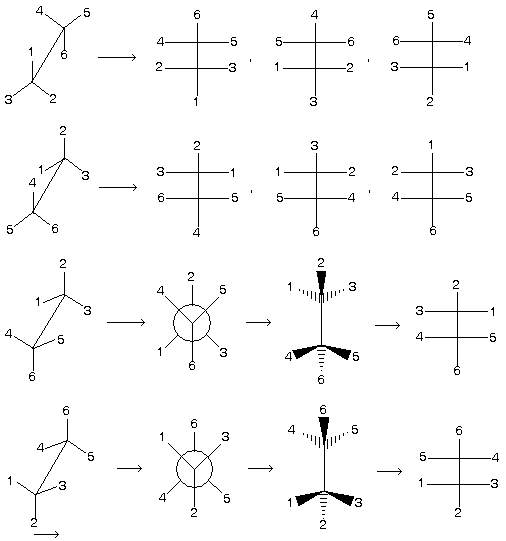
Note here that staggered conformation cannot be directly converted into fischer projection formula because it has always vertical line with away bond group so first change staggered form in appropriate manner followed by inter conversion.
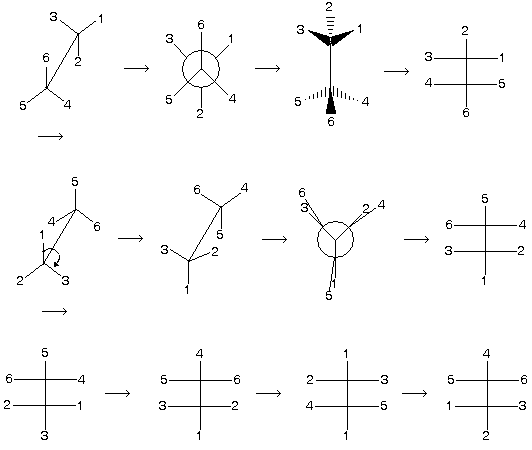
R/S nomenclature
Cahn, Ingold and Prelog gave following rules for R/S nomenclature R & S are Latin presentation and here ‘R’ stands for ‘rectus’ means clockwise or right side rotation for light while ‘S’ stands for sinister means anti clockwise or left side rotation for light. These rules are also known as sequence rules.
(1) When different atomic number having atoms of four substituents are directly attached with a C – atom then priority of groups is considered according to their atomic number for example
Four substituents – H, – CH3 – OH, – Br
Priority order – Br > – OH > – H > – OH
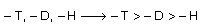
(3) When same atoms are directly attached with a carbon atom, then priority order is given by next atom attach to these atoms so
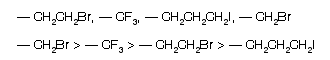
(4) Multiple bonds in the molecules are considered in following way