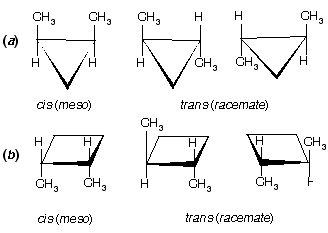Stereochemistry-3
(5) First draw the fisher projection formula of the system, if the group follows clockwise path according to sequence rule, then system is named as ‘R’ and in case of anticlockwise path, the system has ‘S’ name but remember 4th group must be present on vertical line.
(6) If 4th group is placed on horizontal line then opposite names are given to the system.
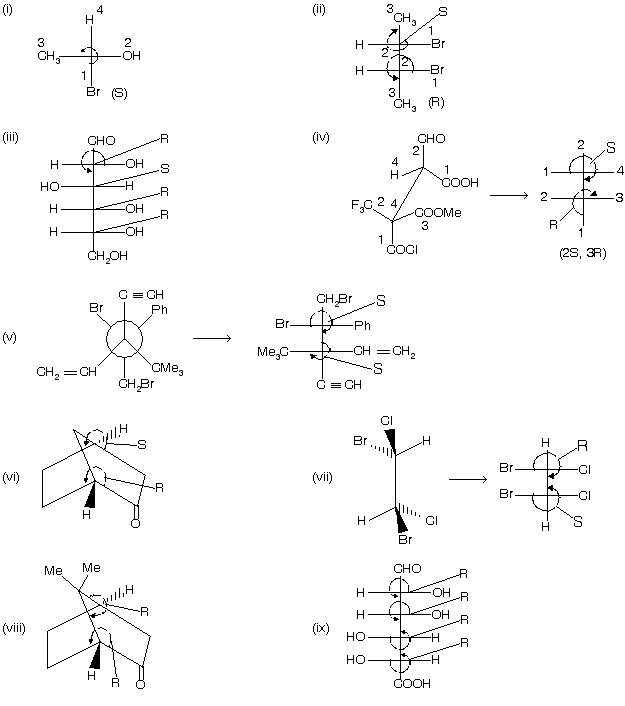
Many other examples are given below where many carbon atoms are present and so we give R/S nomenclature to them.
D/L nomenclature
This is another nomenclature to represent the two different form of optically active compound. Following rules are taken to give D/L nomenclature
(1) Draw the fisher projection formula, taking most oxidised group at the top of the vertical line.
(2) – H and – OH group must be placed on the horizontal line.
(3) If – OH group is present on right hand side of the fisher projection formula, then D – isomer while if – OH group is present in left side than if is L – isomer.
(4) When a compound containing more than one chiral carbon atom, then highest number of chiral centre in a compound is considered for D/L nomenclature.

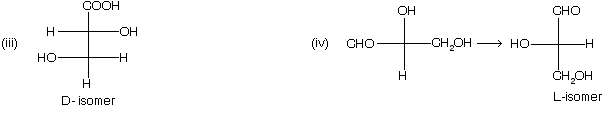
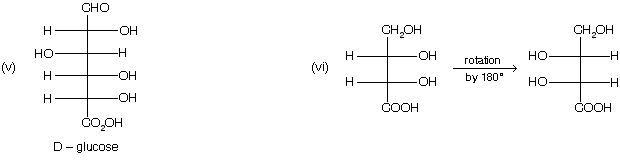
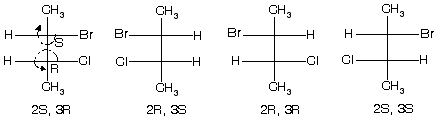
(1) 2-bromo-3-chlorobutane ® optically active isomer
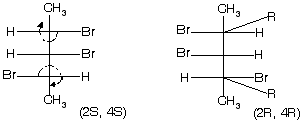
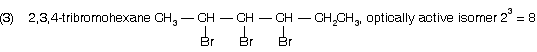

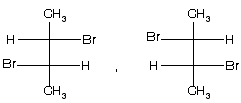
Optical isomerism in alicylic compounds
Prob. How many stereoisomers of (a) methylcyclopropane and (b) methylcyclobutane are possible? Draw them.
Ans. See figure below. One stereoisomer of each; there is a plane of symmetry (dashed line) through the CH3 and H bisecting the ring. Monosubstituted carbocyclic compounds do not have stereoisomers.
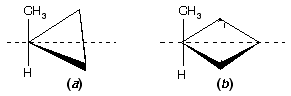
Ans. For (a) and (b) see figure below. Three each: cis-1,2 are meso, and trans-1,2 exist as a pair of enantiomers in each case.