Stereochemistry-4
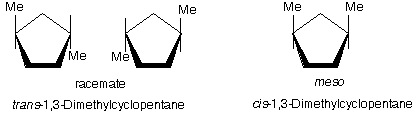
(c) See figure below. Two: cis-1,3 has a plane of symmetry; trans-1,3 has a plane and a center of symmetry. In both isomers C1 and C3 are stereocenters. Switching H and Me converts one stereoisomer to another.
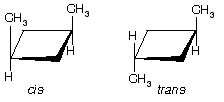
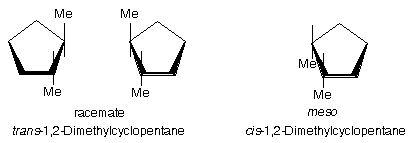
Ans. See figure below. Cyclopentane is best considered as a flat ring. All trans-isomers exist as a pair of enantiomers. All cis-isomers are meso.
Diastereomers show different physical properties, e.g., melting and boiling points, refractive indices, solubilities in different solvents, crystalline structures, and specific rotations. Due to their differences in solubility they often can be separated from each other by fractional crystallization; because of slight differences in molecular shape and polarity, they often can be separated from each other by chromatography. Diastereomers show different chemical properties toward both chiral and achiral reagents. Neither any two diastereomers nor their transition states are mirror images of each other and so will not necessarily have the same energies. The DH‡’s (energy of activation) will be some what different and thus the rates of reaction will also differ. However, since the diastereomers have the same functional groups, their chemical properties are not too dissimilar.
The chemical properties of enantiomers are the same towards achiral reagents, solvents, catalysts, and conditions. But towards chiral reagents, solvents, catalysts, and conditions, enantiomers react at different rates. The transition states produced from the chiral reactant and the individual enantiomers are not mirror images of each other. They are diastereomeric, and they hence have different enthalpies; the DH‡ values are different, as are the rates of reaction and the amounts of product formed. With the exception of rotation of plane-polarized light, enantiomers have identical physical properties. e.g., boiling and melting points, and solubility. (b) The chemical properties are the same towards achiral reagents, but chiral reagents react at different rates. Enantiomers are optically active; the racemate is optically inactive--it does not rotate the plane of polarized light. Other physical properties, such as crystalline form, melting point, and solubility, of an enantiomer and its racemate may differ.
(a) Stereoselective reaction
Reaction in which a single starting material has the capacity to form two or more stereoisomeric product but forms one of them in greater amounts than any of its stereoisomers. Terms such as addition to the less hindered side describe stereoselectivity. This term is more closely connected with structural effect in the reactant as expressed in terms such as addition to the less hindered side.
Addition of halogen on alkene is stereo selective
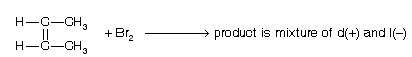
(b) Stereospecific reaction
Reaction in which stereoisomeric starting materials gives stereoisomeric products. Stereospecific is more closely connected with features of the reaction itself than with the reactant. Thus terms such as syn addition and anti elimination describe the stereospecifity of reactions.
A stereospecific reaction can also be stereoselective, for example, syn addition shows stereospecificity in the catalytic hydrogenation of alkenes while the preference for addition to the less hindered face of the double bond describes stereoselectivity.
E2 Elimination is always stereospecific, for trans elimination leaving group –H and –X must be as far apart as possible. Elimination is always anti in nature for example
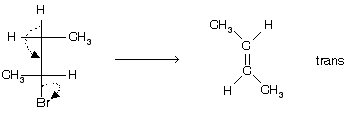
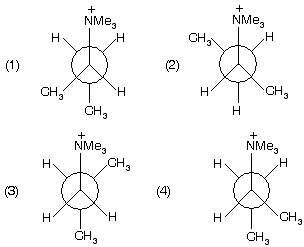
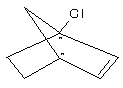
Prob. The most suitable conformation for E2 reaction is ;

(c) Optical inversion
Reversal of the three–dimensional arrangement of the four bonds to sp3 hybridized carbon. It occurs in Nucleophilic substitution of SN2 type also known as walden inversion.
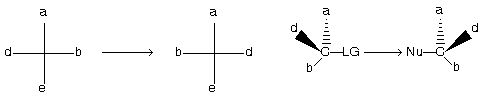
Prob. What is the nature of the products in the following :
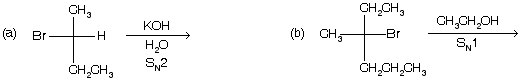
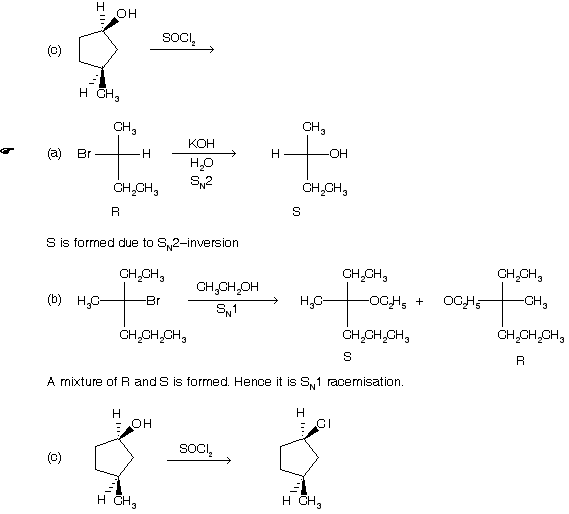
Prob. What do you think about the following compounds — they are having chirality or not.
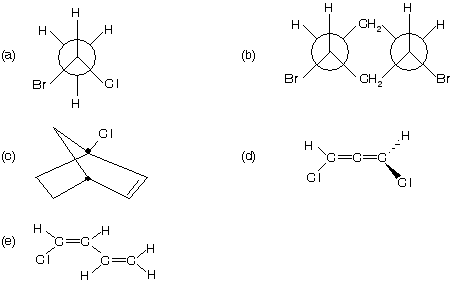

hence it is chiral compound.
(d) There are no chiral carbon, but the molecule is chiral (an allene)
(e) Planar molecule – achiral.
Prob. For each of the following pairs of structures, identify the relation between them. Are they enantiomers, diastereomers, structural isomers, or two molecules of the same compound ?