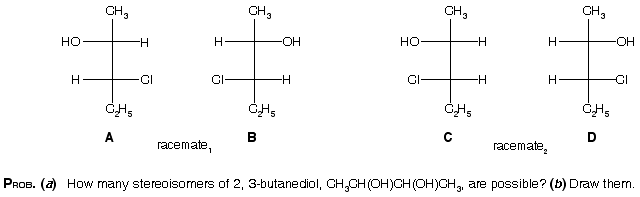
Stereochemistry-5
Ans. (a) There are four stereoisomers. A and B are enantiomers, as are C and D. (b) A and B are diastereomers of C and D.
Ans. (a) We might expect that four stereoisomers are possible (2n = 4). An allowed 180° rotation of A in the plane of the paper gives B; A and B are superimposible and thus identical, and so there are only three stereoisomers. The stereoisomer A or B is called the meso form, and it is achiral and optically inactive. (b) See figure below.
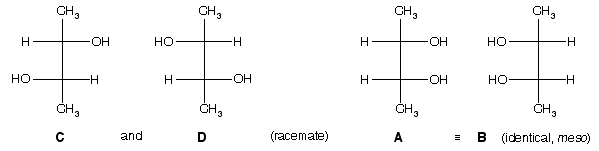
Prob. (a) Why is a meso compound achiral? (b) What structural properties must a meso compound have?
Ans. (a) At least one conformer has either a plane or a point of symmetry, which makes it superimposible upon its mirror image. (See, for example, A and B in Problem) (b) It must have at least one pair of similar stereocenters.
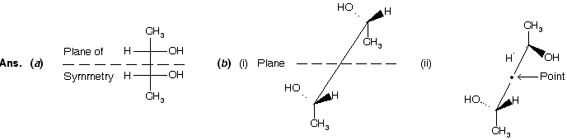
Prob. Draw meso-2,3-butanediol (a) in the Fischer representation showing a plane of symmetry and (b) in the sawhorse representation showing (i) a plane in the eclipsed conformer, and (ii) a point in a staggered conformer.
Prob. Why are the following stereoisomers meso?
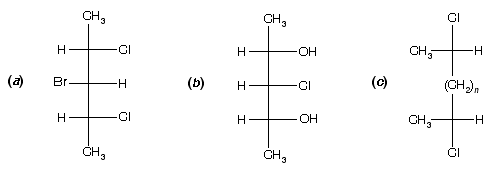
Ans. In (a) and (b), a plane of symmetry cuts through the centre carbon and the two horizontal ligands revealing that the top half of the molecule is the mirror image of the bottom half. It does not matter which ligands are attached to this carbon because they are within the symmetry plane. This is also true in (c) if n = 1 or an odd number. However, if n is an even number, the symmetry plane cuts through the central C¾C bond.
Compounds are optically active mainly due to molecular asymmetry which give rotation to light. Many other examples are also possible which show optical activity (enantiomers) due to molecular asymmetry.
(i) Silicon and germanium containing compounds exist as enantiomers.
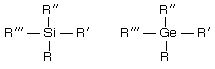
(ii) Quaternary ammonium salts exist as isolable enantiomers
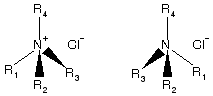
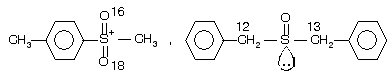
(iii) Chirality of sulphone and sulphoxide due to different isotopes
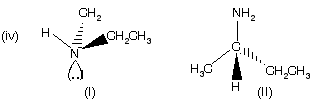
(I) Ethyl methyl amine is a chiral amine but it cannot be resolved due to rapid conversion of lone pair of ‘N’ atom inside out at room temperature (flipping). The best way to picture amine inversion is to compare it to an umbrella in a wind storm. Amine (II) in which the chirality is due to the presence of stereogenic carbon atom can however, be resolved into enantiomers. Very interesting point here that phosphorous containing compound phosphines and sulphur containing compound sulphoxide, sulphonium salt for example
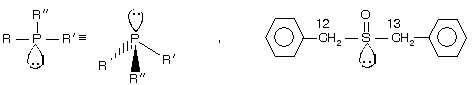
Have the same geometry as 3° chiral amine but these compounds are resolvable because they undergo inversion comparatively very slowly than amines.
(v) Several molecules which do not have a stereo center are however optically active due to molecular asymmetry, they form non-superimposible mirror images.
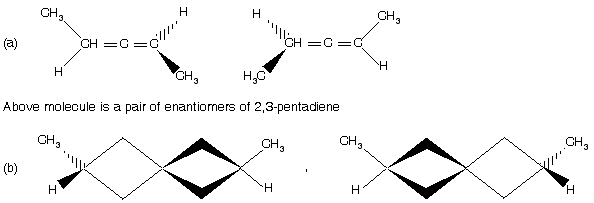
A pair of enantiomers of 2,6-dimethylspiro [3,3]-heptane
These molecules contain chiral axis, where as in the case of allenes, there is a central carbon with sp bonding, 1,2-diene system forces the ligands attached to the terminal of the allene system to occupy mutually perpendicular planes and thus creates molecular asymmetry. The same conditions apply to spirans.
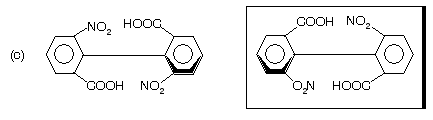
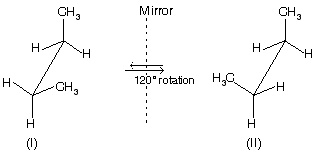
Biphenyls are twisted from the achiral conformation as a result of steric strain between the ortho substituents on adjacent phenyl group. Actually both rings are perpendicular to each other so generation of molecular asymmetry occurs in the molecule which forms mirror images.
Gauche conformation of butane is chiral and structures above are non-superimposable mirror-images of each other, known as conformational enantiomers.
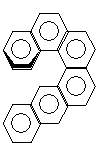
(vi) Several molecules for example hexahelicene gives chirality due to helical shape even though they do not contain stereo centre. However fusion of benzene ring in a linear fashion as in nepthalene, anthracene etc. does not generate chirality. However, if rings are added to produce a curve, the addition of the sixth ring produces a chiral molecule.
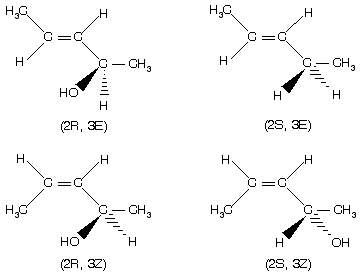
(viii) In case of compounds which contain both a stereocentre and a double bond, for example 3-penten-2-ol exists in four forms (two enantiomers and two E – Z forms).