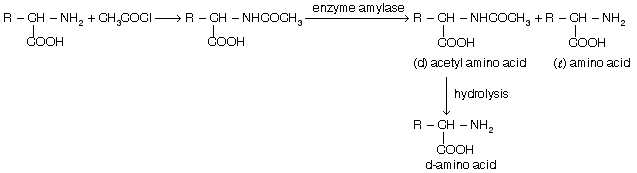Stereochemistry-6
Anti addition of Br2 to Z–2–butene gives a racemic mixture of (2R, 3R) and (25,35), 2,3-dibromobutane through chiral bromonium ion in which olefin geometry is maintained. Either of the two equal approaches of Br-- on the bromonium ion carbons are enantiomeric. The original olefin geometry changes, thus it gives (±) 2,3-dibromobutane.
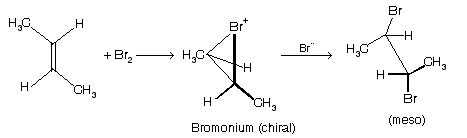
Anti addition form chiral bromonium ion in which geometry of alkene is maintained. Either of the anti attack by Br-- changes the original geometry and thus trans alkenes give meso (optically in active) product.
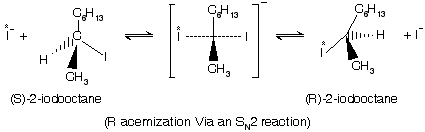
Normally in an SN2 reaction the incoming nucleophile undergo back attack so inversion of configuration with respect to stereo chemistry of substrate. In case the incoming nucleophile and the outgoing leaving groups are same for example when 2-Iodo-octane with sodium iodide, the reaction becomes reversible and an equilibrium is setup between the two enantiomers, leading to recemization, if we do the reaction between optically active 2-iodo-octane and radioactive iodide ions. This is one of the simplest possible types of bimolecular substitution reaction and involves replacement of iodide ion by radioactive iodide ions so that product and starting material are chemically identical.
The cumulated bonding system (compounds with two or more successive double bonds) with an even number of double bonds do not have a plane of symmetry or a centre of symmetry show optical isomerism and must be resolved into enantiomers but cumulative diene with odd number of carbonation shows geometrical isomerism because if allene chain of
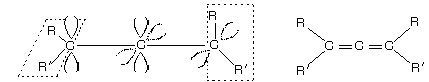
compound is extended by one more double bonds, one then gets a system in which the substituted groups at the two ends of the cumulated chain now lie in the same plane and shows geometrical isomerism.
Prob. Define the terms erythro and threo and illustrate with Fischer diagrams using 3-chloro-2-butanol (CH3CH(OH)CHClCH3).
Ans. These terms are used to describe diastereomers with two chiral C’s (usually adjacent), when both C’s have two sets of identical ligands. In figure below the identical ligands are H and Me. When the different ligands, in this case OH and Cl, are on the same side of the vertical bonds as in figure below (a), the molecule is called erythro (related to the
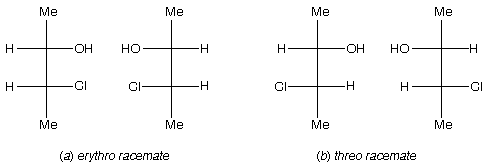
Prob. 2,3-Pentadiene, CH3CH == C == CHCl has no chiral C’s, yet it is a chiral molecule (it has two enantiomers). Explain
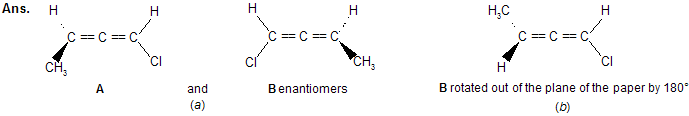
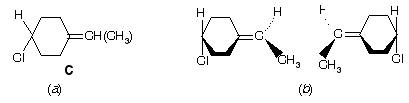
Prob. Draw the two enantiomers of 4-chloroethenylcyclohexane, C, shown in figure below (a).
Ans. This is best done by considering the ring to be flat. (b).
Prob. Spiro compounds are bicyclic compounds having one C common to both rings as shown below. Draw the enantiomers of (a) 2-chlorospiro[4,5]decane and (b) 8-chlorospiro[4,5]decane.
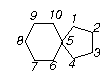
Ans. See figure below. The two rings are in different planes, making the situation reminiscent of the allenes.
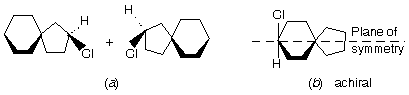
Prob. Write the structures of the three meso diastereomers of C6H12Cl2.
Ans. Here meso structures can be identified easily by their plane of symmetry in at least one of their conformations, which divides them into mirror image halves, each with three C’s. The isomers are:
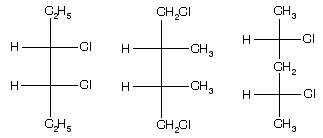
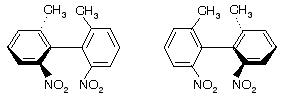
Prob. (a) What is the necessary condition for a pair of conformational enantiomers to be isolable? (b) Draw the enantiomers of the following compound and explain the structural feature which makes their isolation possible.
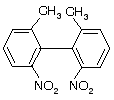
Ans. (a) The rotational energy barrier must be high enough so that interconversion of enantiomeric conformers does not occur. (b) See figure below. This molecule has a high enthalpy of activation for rotating about the bond due to the bulky substituents on the two benzene rings which prevent the rings from slipping past each other. (Note: These substituents prevent the two rings from being flat in the same plane.)
Prob. Point out all the chiral centers in 1-methyldecalin. How many stereoisomers exist?
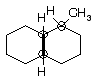
Ans. The three chiral C’s in figure below are encircled, and there are four racemic pairs of enantiomers or eight stereoisomers.
Resolution of racemic mixture
In most of the reactions optically active compounds are obtained in the form of a racemic mixture The method of separation of (+) and (-)forms is known as resolution. The physical and chemical properties of enatiomers are same so they cannot be separated by common methods like distillation as crystallisation. So there are same specific techniques to separate them which are described below:
(a) Mechanical separation:
This is the oldest method used to separate racemic mixture. Pasteur (1948) was the first person to use this technique. This technique is based on the fact that crystals of enantiomers are not same, but they are mirror images of each other. These crystals can be separated under powerful microscopes with the help of forceps.
(b) Biochemical method :
This technique was first of all used by Pasteur. This technique is based on the fact that some micro-organisms like yeast, fungi, bacteria etc destroy one form, from the racemic mixture. The other form is left behind , so it can be separated very easily.
For example :- If mould penicilliam qlaucam is grown on racemic mixture of ammoniam tartrate, pencilliam destroys d-ammoniam tartrate but l - ammoniam tartrate is left behind in the mixture. This can be separated by crystallisation.
(1)The selection of appropriate strain of bacteria is very difficult.
(2)Bacteria cultivate in very dilute solutions so it takes more time to separate the substance by crystallisation.
(3) Bacteria destroy one form from the mixture so we get only one form by this method hence it is costly.
This method is used for the separation among first of all the of acids. Racemic mixture of amino acids is acetylsed then it is left with yeast. Yeast produce an special enzyme known as amylase. It acts as a catalyst in the process