Stereochemistry-7
(c) Chemical method
This is the best method used for the separation of racemic mixture. This was used by Pasteur in 1858. This method is generally used for the separation of optically active acids and bases. If the racemic mixture is acidic it is reacted with optically active base and if it is basic it is reacted with optically active acids to form salts As the solubilities of these salts differ from each other so they can be separated by crystallisation. This method is also known as salt formation method.
Example:
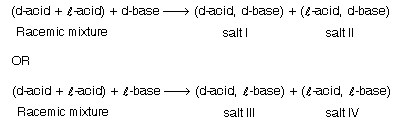
These salts are hydrolysed and the acid is liberated. This method is also used for alcohol, aldehydes, ketones etc.
(d) Column chromatographic method
This is the modern technique which is now used very frequently. Henderson and Rule (1939) used this method for the separation of camphor derivatives. This method is based on the principle of chromatagraphy. According to it, the rate at which a molecule flows through the column adsorbent material is dependent on the shape and size of the molecule.
We pour the racemic mixture on a column filled with the adsorbent material. One form is readily adsorbed at the top of the column but the other goes down, later both these forms can be separated very easily.
Conformational Isomers
The isomers which differ in the conformation are known conformational isomers. This type of isomerism is found in alkanes and cycloalkanes and their substituted derivatives.
(a) Conformation of alkanes
According to Kemp and Pfitzer there is restricted (but not free) rotation about a single bond. The restricted rotation leads to the existence of a single compound of one configuration into more than one spatial arrangements (conformations). So now conformation may be defined as the term used to denote any one of the infinite number of spatial arrangements of the atoms of a molecule that can arise from rotation about a single bond.
(b) Difference between conformation and configuration
The term conformation should not be confused with the configuration which relates to those spatial arrangements of the atoms of a molecule that can be changed only by the breaking and making of bonds whereas the spatial arrangements in conformation are changed simply by rotation about a single bond. In other words we can say that the various stereoisomers that differ in configuration can be inter converted only by the breaking and making of bonds whereas the conformational arrangement may be inter converted by rotation of one part of the molecule with respect to the rest of the molecule about a single bond joining these two parts. The various conformations of some of the important compounds are dealt below to clarify the point.
(c) Representation of conformation
Conformation of alkanes can best be represented with the help of Newmann projection formulae since eclipsing (overlapping or crowding) of hydrogen atoms can best be represented by this formula. In the Newmann projection formula, the carbon atoms nearer to the eye (i.e., the front carbon) and the groups attached to it are represented by equally spaced radii and the distant carbon atom (i.e., the carbon atom farther from the eye or the rear carbon) and the groups attached to it are represented by a circle with three equally spaced radial extensions.
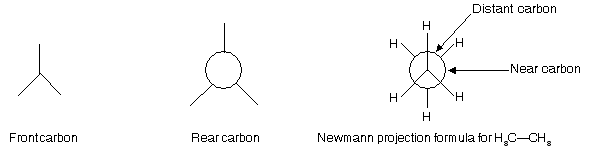
(d) Conformations of Ethane
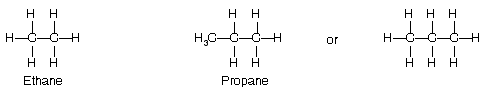
The conformation of ethane, H3C—CH3 affords the simplest possible introduction of the subject. Imagine that one of the methyl groups is rotated along the C—C axis keeping the rest of molecule undisturbed methyl group are possible, each of these possible arrangement represent a conformation.
However, for the sake of convenience each rotation is done in the instalment of 60°. Thus in such case we will obtain six different conformations of ethane. Of these six conformations, the two extreme ones are worth studying because these are very much different from each other, while the intermediate four conformations are almost similar to each other but of course different from the two extreme forms. Thus in short there will be three conformations of ethane, the two extremes and the third one will be the intermediate of the two extremes.
1. One extreme conformation will be such in which the rear methyl group is completely eclipsed by the front methyl group and thus only the front methyl group, i.e., three hydrogen atoms of the methyl group nearer to the eye are visible. Such conformation is known as eclipsed conformation. In the Newmann projection formula of the eclipsed form, the hydrogen atoms are crowded, i.e., the two hydrogen atoms come close to each other.
2. Another extreme conformation will be such in which the rear methyl group has been rotated upside down and thus both the methyl groups, i.e., all the six hydrogen atoms are visible and are as far apart from each other as possible. Such conformation is known as staggered (most stable).
3. The infinite number of possible intermediate conformations between the two extreme conformations are referred to as skew conformations. In these conformations, hydrogen atoms are closer than in staggered but away than in eclipsed conformation.
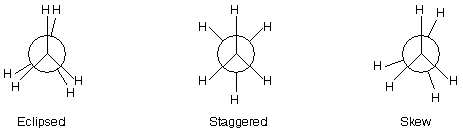
It important to note that all the above conformations of ethane are not equally stable. Among the infinite number of conformations the staggered conformation in which hydrogen atoms are as far apart as possible is the most stable while the eclipsed conformation in which hydrogen atoms are perfectly eclipsed is the least stable; stabilities of the skew conformations lie in between these two extreme limits. Hence the relative stabilities of the various conformations of ethane are in the following order.
This stability order can be explained in terms of repulsive interactions (non–bonded) between bonding pairs of electrons, i.e., electrons pair which form six C—H bonds in ethane. In the staggered conformation, the electron clouds of six carbon–hydrogen bonds are as far apart as possible with the result there is minimum repulsive interactions between these electron clouds. Hence this conformation is quite stable. On the other hand, in the skew and eclipsed conformations, the electron clouds start coming closer. This causes repulsive interactions which is maximum in the eclipsed conformation where there is minimum separation of the electrons of the six C—H bonds. Hence the potential energies of the skew and eclipsed conformations increase and thus their stabilities decrease which is minimum in case of eclipsed conformation.
The repulsive interaction between the electron clouds which affects the stability of a conformation is known as torsional strain. Of all the conformations of ethane, staggered conformation has the least torsional strain and hence most stable while eclipsed conformation has the maximum torsional strain and hence least stable. Due to torsional strain, certain energy called torsional energy, is required to allow rotation around the C—C single bond. In other words, an ethane molecule having staggered conformation will have to cross an energy barrier equivalent to the torsional energy for being converted into eclipsed conformation.
The energy difference between the staggered and eclipsed conformations of ethane is found to be 2.8 kcal/mole which constitutes the energy barrier to rotation about the C—C bond, i.e., for the conversion of staggered to eclipsed conformation. However, this energy barrier of 2.8 kcal/mole is too small for either form to remain stable, i.e., the two forms are inter convertible because even at ordinary temperature, ethane molecules have an average energy 15–20 kcal/mole which can easily overcome the small barrier of 2.8 kcal/mole. This implies that rotation about the carbon–carbon single bond in ethane is almost free for all practical purposes, and it is not possible to separate the different conformations of ethane. Remember that ordinarily, ethane is mostly in staggered form.
(e) Conformations of Propane
Propane can be regarded as derived from ethane by replacing its one of the hydrogen atoms by a methyl group. Since it has two C—C bonds, rotation about C—C single bonds. However, like that of ethane, rotation about C—C single bond is almost free and difference in energy between staggered and eclipsed forms is 3.3 kcal/mole. Again since energy difference is too small to permit a separation of the two extreme conformations of propane, propane molecule exists mostly in the most stable staggered conformation; eclipsed conformation again is the less stable.
(f) Conformation of n–Butane
n–Butane may again be considered as a derivative of ethane whose one hydrogen on each carbon is replaced by a methyl group.
(g) Staggered conformations
From our previous discussion we know that staggered conformations have lower torsional energies and hence are more stable than eclipsed conformations. In case of n–butane three staggered conformations are possible.
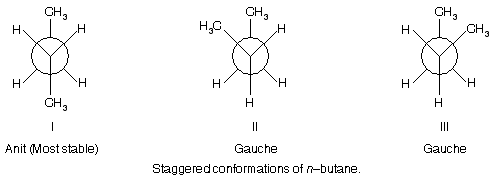
Here it is important to note that the two gauche staggered conformations II and III are non–superimposable mirror images of each other, therefore, they are known as conformational enantiomers. Similarly, like other stereoisomers the pair comprising of the anti form I and the gauche form II or III are termed as conformational diastereomers.
From the energy study of these three conformations, it is found that the anti conformation, I is slightly more stable than the gauche conformations II or III only by 0.9 kcal/mole. Such staggered conformations which either do not differ or differ
slightly in energy from each other are termed as conformational isomers or conformers. Thus here I, II and III conformations of butane are conformers. It is important to note that the conformational isomers are readily inter convertible and hence cannot be separated (difference from the conventional stereoisomers).
(h) Eclipsed conformations of n–butane
Like that of three staggered conformations, there are three eclipsed conformations of n–butane.
In the fully eclipsed form IV, a methyl group is eclipsed by another methyl group, while in eclipsed forms, V and VI, each of the methyl group is eclipsed by hydrogen. Of these two types of eclipsed conformations, the one having larger methyl groups eclipsing, i.e., IV, will naturally feel more repulsive force than the V and VI where a methyl group is eclipsed with a hydrogen atom. Thus out of these three conformations, IV is less stable while V and VI are more stable.
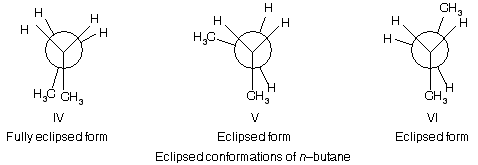
(i) The fully staggered form (anti form) I is more stable than the fully eclipsed form IV by about 5.3 kcal/mole (22 kJ/mole).
(ii) The anti form I is more stable than the gauche forms II or III by about 0.9 kcal/mole (3.8 kJ/mole).
Thus the relative stabilities of the six conformations of n–butane can be represented as below.

Thus at any time, n–butane consists of an equilibrium mixture of all possible conformations, of course with the highest proportion of the anti form, I and the least proportion of the fully eclipsed form IV.
Cyclohexane has two conformations, e.g., boat and chair form. Chair form is more stable than the boat form by 44 kJ mole-1.
It should be noted that even though different conformations of a compound cannot be separated, the study of conformations known as conformational analysis proves quite useful to the chemists. This is because stabilities and shapes of different conformations are of considerable help in understanding the properties of compounds.