The Concepts of Chemical Equilibrium for IIT-JEE
History of chemical eqm started from At beginning of 18th century whole idea of chemical eqm. is based on there basic ideas: in complete reaction, reversibility & dynamic. Berthollet, Clande Laus (1748-1822) was pioneer in this field. He tonght chemistry to Napolean & ment to Egypt in 1798. He observed these that high conc. of sod. Carbonate by Lake Neutron on edge of the desert. He conclanded that under prevailing condition, Nice in upper layee of soil hard reacted with calcium carbonate from nearby lime stone hills. From there he started on working of chemical eqm. Chemical Equilibrium gives us the answer that why we cannot have 100% yield in a reaction? This chapter is of prime importance in physical chemistry. If opposing processes involve chemical changes. Chemical Here eqn. is called chemical eqm.
aA + bB  xX + yY
xX + yY
Physical Eqn.:-
1. Solid – liquid Eqn. :- (Meeting office)
| Rate of melting of ice = Rate of freezing of water |
H2O(s) H2O(l)
H2O(l)
Temp. at which solid & liquid form of pure substance are in eqm. atm. Pressure c/d freezing point.
2. Liquid – Gas eqm.
(Evaporation of water in closed vessel):
| Rate of evaporation= Rate of condensation |
H2O(l) H2O(g)
H2O(g)
3. Solid – Solution eqm.
:- Dissolution of sugar in water
| Rate of dissolution=Rate of precipitation |
Sugar (Solid) Sugar(insolution)
Sugar(insolution)
Solubility :-
Amt. Of solid in gms. That dissolves in 100g of solvent to from saturated solution at particular temp.
4. Gas - solution eqm. -
(Dissolution of gas in liquid)
Co2 Co2(in solution)
Co2(in solution)
Reversible Rxn. :-
Rxn. which takes place not only in forward direction but also in backward direction. Under same condition
A + B C + D
C + D
PCLS(g) PCL3(g) + CL2(g)
PCL3(g) + CL2(g)
direction
eq. AgNo3(aq.)+NaCl(aq.) AgCl(s)+NaNo3(aq.)
Concept of chemical Eqn. :
A + B C + D
C + D
At eqm.
| Rate of farward rxn. = Rate of backward rxn. |
CaCO3(S) CaO(s) + CO2(g)
CaO(s) + CO2(g)
Characteristics of chemical Eqm.:
1. At eqm. , conc. of each of reactants & products becomes constant.
2. At eqm. , rate of forward rxn. , becomes equal to rate of backward rxn. So, eqm. is dynamic in nature.
3. A chemical eqm. can be established only if none of products is allowed to escape out or separate out as a solid.
eq. AgNo3+KCl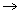 AgCl
AgCl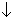 + KNo3
+ KNo3
4. Chemical eq. Can be attained from either direction
eq. N2O4(g) 2NO2(g)
2NO2(g)
(Colourless) (Raddish brown)
5. Catalyst does not alter state of eqm.
Question: why catalyst does not alter state of eqm.
Answer: Catalyst speed of forward as well as back was reaction to same extent. So, eqm. is not disturbed.
Law of Mass Action:-
Rate of rxn. is proper to product of active masses of reactants, each raised to power equal to its stoichiometric coiff. as in balanced equation
Law of Chemical Eqn.:-
A + B C + D
C + D
Applying Law of mass action.
Rate of forward r*nk=![]()
Rate of backward rxn. a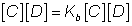
At eqm.
Rate of forward rxn. = Rate of backward rxn.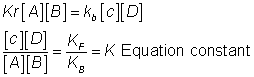
aA + bB xX + yY
xX + yY
By law of Mass action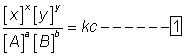
for gas Phase reaction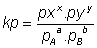
Relation b/w kp & kc
aA + bB xX + yY
xX + yY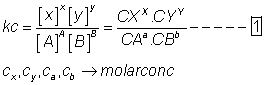
if x,y, A &B are gaseous, then![]()
If gases are ideal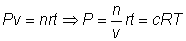
Q.why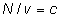 ?
?
ans:- n/v =no. of moles/litre=c for gases A,B,X & Y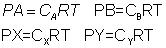
Putting these values in eq (ii)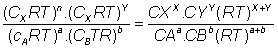
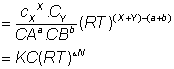
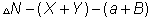
= no. of moles of reactants.
Illustration
:- At 773k, eqn constant kc for r*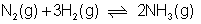
is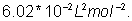
.
Calculate value of Kp at same temp.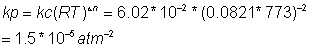
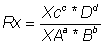
where xc, xD,xa& xb
are margin
partial pressure= Molefraction xTotal pressure (pT)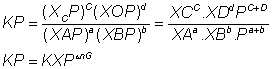
NOTe: 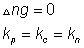
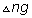
=no. of moles of product(gas)-no. of moles of reactant(gas)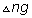
Take molar conc. & pressure of solids as units.
Characteristics of Eqm. Constant:
(1) Value of eqm. constant for given rxn. is always constant depending only upon temp. of rxn. is independent of conc. of reactants with which me start or direction from which eqm. is approached.
(2) If rxn. is reversed, value of eqm. constant is reversed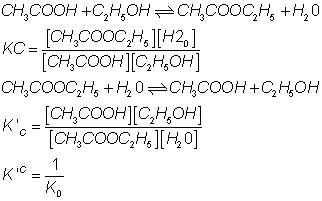
for new eq. Is square root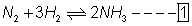
then for rxn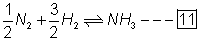
value of eqn. constant k'=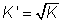
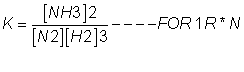
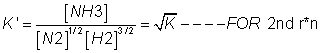
4. if eq.(having eqn constant k) is written in two steps (having eqn constant k1 &k2) then k1xk2=k
N2+ 2O2  2NO2
2NO2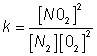
N2+ O2  2NO
2NO
2NO + O2 2NO2
2NO2
for 1st step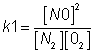
for 2nd step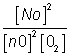
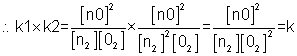
(V) Value of eqm. constant is not affected by catalys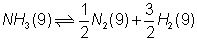
for reverse reaction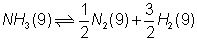
dividing by two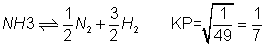
Thermodynamics of chemical equilibrium
for a process at constant T and p if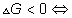
process is spontaneous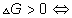
process s spontaneous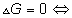
process is equilibrium
Dumb quesstion: what is g?
sol:- g is gibbs free energy and is defined as G=h-ts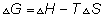
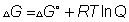
where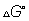
is the value of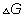
at standard condition Q= reaction quotent
Q: what is reaction quotient?
sol:- For areaction aA + bB xX + yY
xX + yY
at any time r.Q =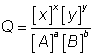
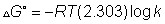
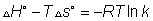
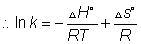
Differenteat both sides wrt T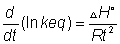
Nant haff's equation
at two different temperature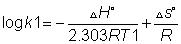
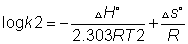
So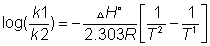
For reverse rxn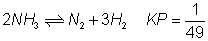
on dividing by 2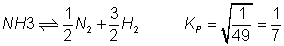
eq. H2(g) + I2(g) 2HI(g)
2HI(g)
Fe3+(aq.) + SCN(aq.) [Fe(SCN)]2T(aq.)
[Fe(SCN)]2T(aq.)
Heterogeneons Eqn.:-
When reactants & products arepresent in two or more than two phasees.
eq. CaCo3(s) CaO(s) + Co2(g)
CaO(s) + Co2(g)
Writing Expression for Eqn. Constant(k):-
[ Pure solid ] = 1 & [ Pure liquid ] = 1 (if present as solvent)
For Hetrogenous Eqn.:-
(i) H2(g) + I2(g) 2HI(g)
2HI(g)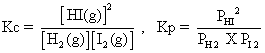
(ii) PCls(g) PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)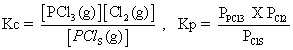
For Hetrogeneous Eqn.:-
CaCO3(S) CaO(S) + CO2(g)
CaO(S) + CO2(g)
By law of chemical eqn.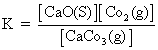
By conversion, Putting [ CaO(S)] = 1 & [CaCo
3
(S)] = 1
K = [CO2(g)]Kp= PCO2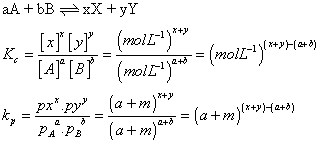
Application of equation constant
(1)
Predicting extent of r*n
:-
(a) larger value of eqn. constant
shows that forward
rxn is favoured.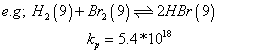
(b) intermediate value of k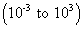
shows that conc. of reactants & products are comparable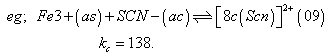
(c) low value of shos that backward rxn is favoured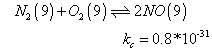
2.predicting direction of rxn:-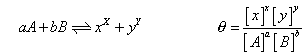
(1) If Q = k, rxn. is in equetion
(2) If Q > k, Q will tend to so as to becomes equal to 1.so, rxn. will proceed in backward direction.
(3) if Q < k, Q will tend to increase, So rxn. will proceed in forward direction.
Versal & when eqn. was dessociated into PCl3& Cl2. Calculate eqn. constants Kp& Kcfor this rxn.
Ans
PCl5 PCl3+ Cl2
PCl3+ Cl2
% dissosiation of PCl5at equation = 40%
Amount of PCl5, PCl3& Cl2
at equation will be
PCl5= 2-0.8 = 1.2 mole, PCl3= 0.8mole & Cl2= 0.8 mole.
Domb qouestion: [{Q. why mole of PCl3& Cl2are 0.8 ]
Ans. 1 mole of pcls on dissociation gives 1 mole of PCl3& 1 mole of Cl2 Vol. of container = 2L
molac conc. at equation will be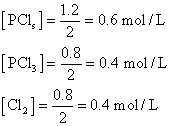
By law of chemical equation.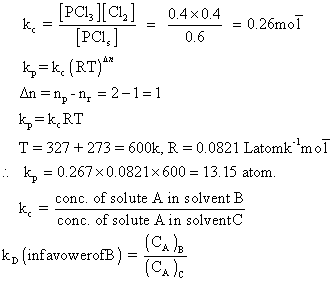

Illustration
:- Ratio solvelity of oxialic acid in H2O and ether is 10. 1 L of eq. Sol. Of oxalicacid in H2
O i0 g/L. was mixed with 100 ml of ether Calculate conc of oxalic acid in g/L in ether long.
Ans. Let x gm of oxalic acid present in either them(10 - x)g is present in H2O.
Ko=10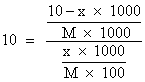
on solving x = 0.0999 g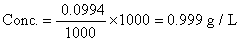
Deg. of dissociation in term of v.d
:-
Using the results of solutions chapters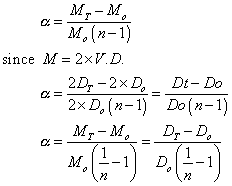
Illustrartion
: 0.0753 g of selenium vapours occupiedmol of 78ml at STP. Selenium is in state of equation.
Se6(g) 3Se2(g)
3Se2(g)
Calculation deg. of dissociation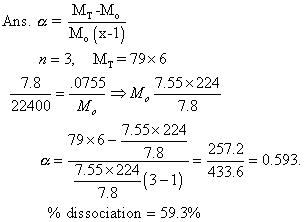
Factors affecting equation:-
(1) Chyange of conc. Of any reactant or product
(2) Change in temp. of system
(3) Change in pressure of system
(4) Tradition of catalyst
(5) Addition of inert gas.
Effect of addition of catalyst
:- Addition of catalyst does not disturb eqn. But helps in attainment of eqn. quickly.
(a)
If rxn takes place at constant vol.(closed vessel)
:- Addition of inert gas will not chang mole conc. Of reactants & products. So, state of eqn. Will remain unaffected.
(b) If rxn. Takes place at constant pressure, Hence dissociation of pcl3 increases.
Dumb quation:-[Q. How this happen?
Ans. Since p is cnstant, so addition of inert gas will Total volume. So, at equation, molec conc of each reactant old products will . So,kc is constant at constant temp.
So, to keep kc constant either [PCl5]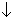 should or [PCl3] & [Cl2] should
should or [PCl3] & [Cl2] should . This happen only if PCl5dissociates.
. This happen only if PCl5dissociates.
Le chathelier's principle:-
If a in eqn. Is subjected to a change of conc. Temp. of pressure, eqn. Shifts in direction that tends to undo the of the change imposes.
(1) Effect of conc.:-
A + B C + D
C + D
If rxn is in eqn. Mole of A or B is added, then ,according to Le chatelic's principle effect will be to
conc. Of A & B react to frome C & D. I,e;eq m. shifts in forward direction. Shift in forward.
(2) Effect of temp.:-
Every rxn. is made up of two opposing rxns. If to rewards rxn. Is exothermic, then backwardrxn.![]()
If temp. is
,I,e; heat is supplied to system; according to the chateliec princple. i,e; eqn. Will shift to side heat absorbs heat I,e; in backward direction & vice versa.
Note
:- Exothermic rxn are forward by low temp. whereas end. thermic rxn. are favoured by high temp.
(3) Effect of pressure:-
Significant role to play only in case of gaseons rxn.
N2O4(g) 2NO2(g)
2NO2(g)
1 mole 2 mole
Forward rxn. Occurs with
in no. of moles & viseversa. If pressure on system
, then according to Le chateliec's principle , eqn. Will shift in direction in which pressure
or no of moles (b/c pressure is directly proportional to no. of moles). Since backward rxn. Takes place with in no of. moles. So,
in pressure will favovr. Combination of NO2molecules to N2O4.
Note
:-
(1) Low pressure tavours those rxns which accompany by
in total no of moiles & vice versa.
(2) Pressure has no effect on eqn. Rxn which proceed which no change in total no. of moles. So,
in volume will shift eqn. In direction in which no of moles decreases.
I,e; eqn. Constant does not change with temp.
(ii)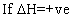 log(k2/k1)= +velog k2>log k1
log(k2/k1)= +velog k2>log k1 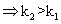 I,e; eqn. Constant
I,e; eqn. Constant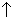 with
with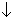 in temp.
in temp.
(iii)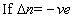 log(k2/k1) = -velog k2< log K1
log(k2/k1) = -velog k2< log K1 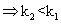 I,e; eqn. Constant
I,e; eqn. Constant with
with in temp.
in temp.
Q.1 Calculate eqn. constant for rxn.
H2(g) + CO2(g) H2
H2
O(g) + CO(g) at 1539K, if eqn. constants at 1395K for following are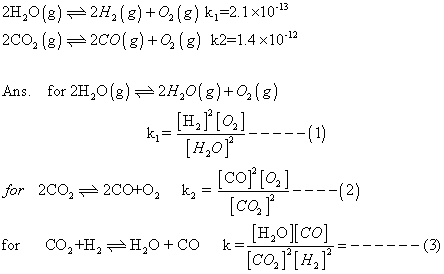
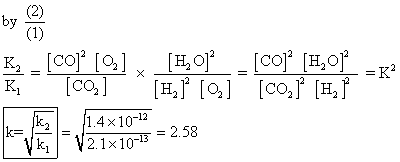
Q2.for reaction
A + B 3C at 290
3C at 290
C, 3L vessel contains 1,2,4 mole A, B & c resp. predict direction of rxn .
if (a)kcfor rxn. is 10
(b) kcfor rxn is 10.66
Ans.
:- A + B 3C
3C
Before rxn; [A] = 1/3
[B] = 2/3
[C]= 4/3
so,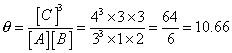
and this [c] must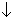
or [A] & [B] should so, rxn mole occurs in backward direction
(b) Q = Kc
, So, rxn. is in eqn.
Q.3 At temp, 2AB2(g) 2AB(g) + B2(g)
2AB(g) + B2(g)
with deg association 'x' which is small compared to unity. Device expression for 'x' in terms of eqns. Const. Kp& total pressure P.
Ans. 2AB2(g) 2AB(g) + B2(g)
2AB(g) + B2(g)
Mole after dissociation
Mole after dissociation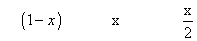
Total mole at eqn.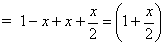
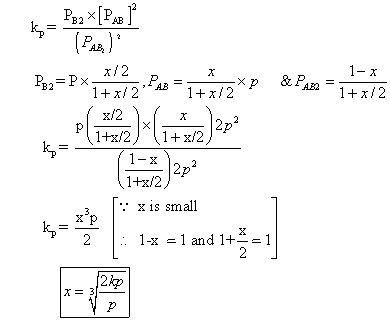
Q.4:- kCFOR a2(G) + b2(g) 2AB (g) at 1000
2AB (g) at 1000
C is 50. If one line flask containing 1 mole of A2 is connected with a 2L flask containing
2mole of B2 ; How many mole of AB will be formed at![]()
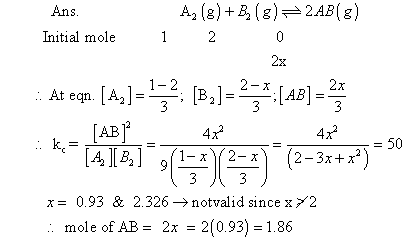
Q.5 N2(g) + O2(g) 2NO(g)
2NO(g)
was established with an eqn. constain K
c= 2.1 X 10- 3. At eqn. mole % of No. was 1.8. estimates initial composition of air in mole fraction of N2& O2.
Ans: N
2
(g) + O2  2NO
2NO
initial a (100 - a) 0
final (a - x) (100 - a - x) 2n
given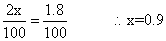
also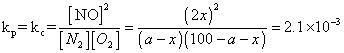
a = 79%, 100 - a = 21%
Q.6: kpfor rxn. N2+ 3H2  2NH3is 1.6 X 10-4atm-2at 4000c what will be kpat 5000
2NH3is 1.6 X 10-4atm-2at 4000c what will be kpat 5000
C ? Heat of rxn. in this temp. range is - 25.14 KCal.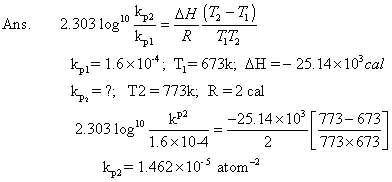
Out Kpfor rxn. Also report Kp& G0forN2+ 3H2
G0forN2+ 3H2  2NH3at 250C
2NH3at 250C
Ans. - G0= 2.303 RT log10Kp- ( - 16.5 X 103) = 2.303 X 8.314 X 298 log10Kp
G0= 2.303 RT log10Kp- ( - 16.5 X 103) = 2.303 X 8.314 X 298 log10Kp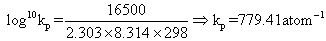
Kp1for N2+ 3H2  2NH3Kp1= (Kp)2= (779.41)2= 6.07 X 105atm- 1Also
2NH3Kp1= (Kp)2= (779.41)2= 6.07 X 105atm- 1Also G01= 2.303 X 8.314 X 298 log10(6.07 X 105) J
G01= 2.303 X 8.314 X 298 log10(6.07 X 105) J
=32.998 KJ G01= -32.998 KJ
G01= -32.998 KJ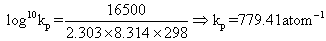
Q.9 At S4 Ok & 1 atom, N2O4is 66% dissociated into NO2
what mol. of 10g N2O4occupy under these condition
Ans:- N2O4 2NO2
2NO2
initial 1 0
at eqn
total mole at eqn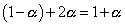
but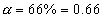
Total mole of eqn. = 1 + 0.66 = 1.66
1 mole of N2O4
is taken, mole at eqn.=1.66![]()
mole of n2o4 is taken ,mole at eqn. =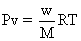
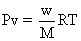
1 X v = 0.18X0.0821X340 = 5.02 L
Q.10 heat of rxn for rxn. At constant vol. is 1200 cal more than at constant pressure at 300k. calculate ratio of eqn. Constant kp& kc.
Ans.  E =
E = H = 1200 Cal. Whrere
H = 1200 Cal. Whrere E is heat of rxn. at constant volume &
E is heat of rxn. at constant volume & H is heat of rxn. at constant pressure
H is heat of rxn. at constant pressure H =
H = E +
E + nRT
nRT nRT = -1200
nRT = -1200 n = -1200 / 2X300 = -2
n = -1200 / 2X300 = -2
Kp= Kc(RT) n
n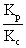 = (0.0821 X 300)-2= 1.648 X 10-3
= (0.0821 X 300)-2= 1.648 X 10-3
Q.1. 0.96g of HI sere heated to attom eqn. 2HI H2+ I2
H2+ I2
rxn. mixture on titration required 15.7 ml of N/10 hypo sol. Calculate deg. of dissociation of HI.
Ans. 2HI  H2+ I2
H2+ I2
mole at t = 0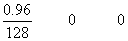
= 7.5 X 10
-3
at eqn.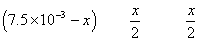
meg. Of I2
formed at eqn. = meg.of hypo used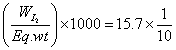
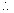 Eqn. of I2
Eqn. of I2
=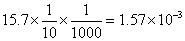
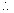
Mole of I
2
formed =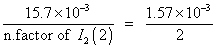
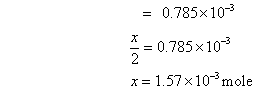
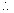 Deg. of dissociation of HI =
Deg. of dissociation of HI =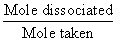
=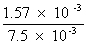
= 0.209 = 20.9 %
Q.2 An eqn. Mixture of
CO(g) + H2O(g) CO2(g) + H2(g)
CO2(g) + H2(g)
present in a vessel of 1 L capacity at 815c was found by analysis to contain 0.4 mole of Co, 0.3 mole of H2O, 0.2 mole of Co2and 0.6 mole of H2
(a) Calculate kc
(b) To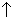 conc. to 0.6 mole by adding co2to vessel, how many mole must be added into eqn mix at mole at eqn 0.4 0.3 0.2 0.6
conc. to 0.6 mole by adding co2to vessel, how many mole must be added into eqn mix at mole at eqn 0.4 0.3 0.2 0.6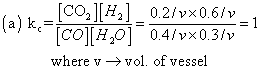
(b) Suppose 'a' mole of CO2 are forced in vessel at eqn.; by doing so rxn proceeds in backward direction
i.e Co2+ H2  CO + H2O
CO + H2O
at intial time (0.2 + a ) 0.6 0.4 0.3
at eqn. (0.2+a-0.2) (0.6-0.2) (0.4+0.2) (0.3+0.2)
a 0.4 0.6 0.5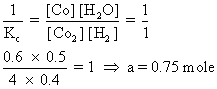
Q.3 Deg of dissociation is 0.4 at 400k and 1 atom for gaseous rxt PCl
s  PCL3+ CL2
PCL3+ CL2
Assume ideal gas calculate density of eqn mixture at 400k and 1 atm.
(Atomic mass of p=31& cl=35.5)
Ans: PCl
s  PCL3+ CL2
PCL3+ CL2
intial mole 1 0 0
At eqn. (1-0.4) 0.4 0.4
Total mole at eqn. = 1 - 0.4 + 0.4 + 0.4 = 1.4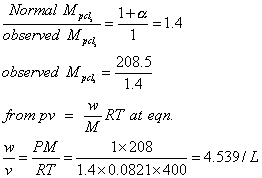
Q.5 Heat enthalpy standard free energy change for
eqn. Zn(S) + H2O(g) ZnO(s) + H2(g)
ZnO(s) + H2(g)
eqn Are 224 kJ mol
-1
and 33kjmol respectively at temp 920 calculate temp at which eqn constant becomes greater than
(1) Assume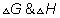
remains constant.
Ans.  G0= -2.303 RT logK
G0= -2.303 RT logK
33000 = -2.303 X 8.314 X 920 log k
k920k= 1.34 x 10- 2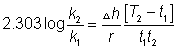
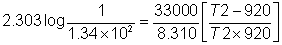
T2= 1078.5 K.
SO, At any temp above this k will be greater than
Hard type
Q.1:- Solid nh4 on rapid healthy in aclosed vessel at 357 develop a constant pressure of 275 mmHg owing to partial decomposition of NH4I into NH
3& HI . calculate final pressure developed at eqn.kpfor dissociation of HI is 0.015 at 357 C
Ans:- NH4I(g) NH3(g) + HI(g)-----(1)
NH3(g) + HI(g)-----(1)
for this eqn 2p=275 --> p=137.5
So kpfor eq. (1)
Kp= PNH3XPHI= 137.5 X 137.5 ---------(2)
For 2HI H2+ I2
H2+ I2
1 0 0
(1 - x) x/2 x/2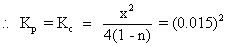
Dumb question
: why we have taken kp (0.015)2?
Ans: K
pfor division of HI = 0.015
HI
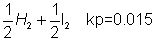
K
p
= 0.015
2HI H2+ I2 Kp= (0.015)2x = 0.2
H2+ I2 Kp= (0.015)2x = 0.2
Again since HI decomposes rxn proceeds in forward directioal (by Leehatetics Principal)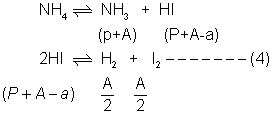
But a = (P + A) X 0.2
also kp
for final eqn (3)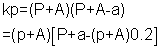
= 0.8(P + A)2But Kp
= 137.5 X 137.5
0.8(P + A)2
= 137.5 X 137.5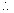 P + A = 153.73
P + A = 153.73 A = 16.22
A = 16.22
a = (p + A) X 0.2 = 153.73 X 0.2 = 30.75
Total pressure at eqn =PNH3+PHI+PH2+PI
=(P+A)+(P+A-a)+A/2+A/2
=2p+2A
=2(p+A)= 2x153.73
=307.46 mmHg
Q.2: when 1 - Pentyne (a)is treated with 4n atc. KOH at 175 it slowely get converted into an eqn mix of 1.3 % 1 pentyne 95.2% 2 pentyne(b) of 1,2 pentadiene (c)eqn. was maintained at 175c calculate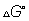
for following eqn.
from calculated value of g1 and g2 indicate order of (a) (b)(c) write a reasonable rxn mechanism in showing all intermediates reading to (A)(B)(C)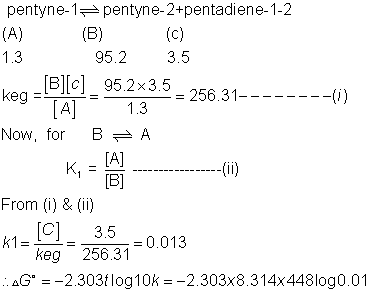
= 16.178 KJ
Stability order for A & B is B > A
Similerly for B C
C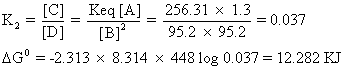
So, stability order for B and Cis B > C
Total stability order is B><>A
Q3. N2& 02
combine at a giventemp to produce no. at eqn the yield of no is x% by volume. If x =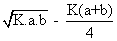
where k is is eqn constant given rxn. at given temp and a and b volume % of n2 &o2 respectively in intial pure mixture what should be the intial composition of reacting mixture in order that max. yield of nois ensured? also repo max value of k at which x is maximum?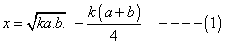
'x' is maximum only when condition of maximum are fulful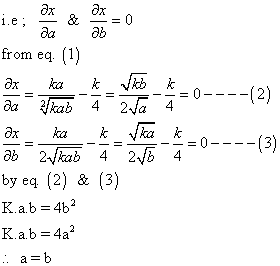
Note: this valid only when k<4 because if a = b Eq.(1)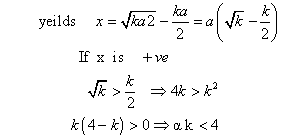
Equilibrium
Physical Eqn.
Low of mass action
Law of chemical Eqn.
Eqn.constant
Revessible rxn.
Forward rxn
Backward rxn
Gibbs free energy and Spontaneity
Homogeneous Equilibrium
Heterogeneous Equilibrium Le chatelier's principle
Source: goiit.com