Acidic Powers & Their Orders - Chemistry Fact Sheet- 1
Acidic Powers & Their Orders - Chemistry Fact Sheet- 1
| 1. | Farther the (–I) group (Cl), lesser the acidic strength | ||
| 2. | Farther the (+I) group, greater the acidic power | ||
| 3. | 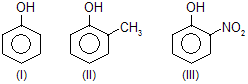 | —CH3 is electron donating and — NO2 is electron attracting | |
| 4. | 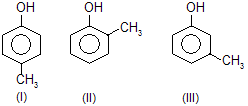 | —CH3 is electron repelling; decreases acidic strength of phenol | |
| 5. | 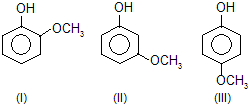 | — OCH3 group contains +M effect and decreases acidic poer. | |
| 6. | 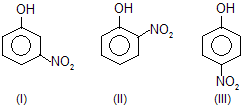 | — NO2 is electron attracting; III is more resonance stabilised than I and also than II. In I, only inductive effect is operative. | |
| 7. | 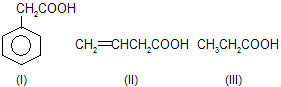 | sp2 hybridised carbon of I, II are more el-ectronegative hence acid strength is inc-reased. Benzylic (C6H5CH2) is more stab-ilised than allylic (CH2==CHCH2). | |
| 8. | 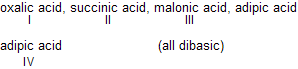 | Effect of one —COOH on the other decr-eases as its distance between them increases, (COOH)2 is maximum acidic. | |
| 9. | —NO2 is electron attracting (–I effect) | ||
| 10. | —OH shows electron withdrawing nature at o - and m - and electron repelling at p -, o - isomer due to intramolecular bonding in salicylate ion is stronger than m - isomer | ||
| 11. | —do— | ||
| 12. | —NH2 is electron donating. |