VITEEE 2009 Sample Question Papers - Chemistry
VITEEE 2009 Sample Question Papers - Chemistry - PART – 2 [CHEMISTRY]
41. The oxidation number of oxygen ¾
Ans: KO 3: + 1 + 3x = 0, x = ![]()
Na 2O2: + 2 + 2x = 0, x = - 1
Choice (D)
42. Reaction of I Cl3 and PhMgBr ¾
Ans: Triphenyl phosphene
3C 6H5MgBr + PCl3 ® (C6H5)3P + 3MgClBr
Choice (C)
43. ……. not a characteristic of transition elements?
Ans: Choice (D)
44. Cl - P - Cl bond ¾
Ans: Arial bonds are 90° and Equatorial bonds are 120°.
Choice (A)
45. --- magnetic moment ¾
Ans: No unpaired electron.
Choice (A)
46. The number of Formula units of calcium fluoride CaF2 ¾
Ans: Number of moles = ![]()
Number of formula units = ![]() ´ 6.023
´ 6.023
´ 1023
= 1.129 ´ 1024
Choice (A)
47. The IUPAC name of the given ¾
Ans: Choice (C)
48. When SCN? is added to an aqueous ¾
Ans: Fe 3+ + SCN? + H2O ® [Fe(H2O)5SCN]2+
Choice(B)
49. Hair dyes contain
Ans: Choice (C)
50. Schottky defects occurs ¾
Ans: Schottky defect occurs when the ions have almost the same size
Choice (B)
51. The number of unpaired electron ¾
Ans: [Co(NH 3)6]3+ - Inner orbital complex
- No unpaired electron
[CoF 6]3? - Outer orbital complex
- 4 unpaired electrons
Choice (D)
52. The standard free energy change of a reaction ¾
Ans: - D G° = 2.303 RT log Kp
![]() log Kp
log Kp
0.02016 ´ 103 = log Kp
= log K p = 20.16
Choice (A)
53. If an endothermic reaction occurs spontaneously at ¾
Ans: For an endothermic reaction D H is positive, the reaction occurs spontaneously when D S > 0
Choice (C)
54. If a plot of log10 C versus t fives a straight line ¾
Ans: t = ![]()
t ´ ![]() = logC0 - logC
= logC0 - logC
logC = logC0 - t ´ ![]()
logC is t in a stline with slope ![]()
Choice (B)
55. A spontaneous process is one in which ¾
Ans: For a spontaneous process D G is negative.
Choice (B)
56. The half life period of a first order reaction ¾
Ans: t ½ = 100 sec.
For a first order reaction t ½ = ![]()
K = ![]() =
= ![]() = 6.93 ´ 10?3 sec?1
= 6.93 ´ 10?3 sec?1
Choice (B)
57. The molar conductivities of KCl, NaCl and KNO3 are ¾
Ans: ![]() =
= ![]()
= 128 + 111 - 152 = 87 S cm2 mol?1
Choice (B)
58. The electrochemical cell stops working ¾
Ans: When both the electrode potentials become equal cell reactions stops.
Choice (B)
59. The amount of electricity required to produce ¾
Ans: 1 mole of copper is 2 equivalents
Current required = 2 Faraday
Choice (C)
60. Dipping iron article into a strongly alkaline ¾
Ans: Choice (C)
61. Hydroboration oxidation of 4-methyl-octene ¾
Ans: Hydroboration – oxidation of alkenes give alcohols containing the same number of carbon atoms. Net reaction involves addition of H2O against Markownikoff’s rule.
Choice (A)
62. When ethyl alcohol is heated
Ans: Ethanol undergoes dehydration when heated with conc.H2SO4 to form ethylene.
Choice (D)
63. Anisole is the product obtained from ¾
Ans: Anisole is phenyl methyl ether.
Choice (B)
64. Ethylene glycol gives oxalic acid ¾
Ans: Choice (C)
65. Diamond is hard ¾
Ans: Choice (A)
66. A Wittig reaction with an aldehyde ¾
Ans: Carbonyl compounds react with phosphorous yields to form alkenes. This is known as Witting reaction.
Choice (C)
67. Cannizzaro reaction is ¾
Ans: HCHO does not contain a -hydrogen atom. So it undergoes Cannizzaro reaction.
Choice (A)
68. 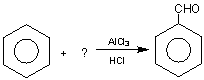 ¾
¾
Ans: C 6H6 + CO ![]() C6H5 - CHO
C6H5 - CHO
This is known as Gattermann-Koch aldehyde synthesis.
Choice (C)
69. Maleic acid and Fumaric acids ¾
Ans: Maleic acid is the cis-isomer and fumaric acid is the trans-isomer.
Choice (B)
70. The gas evolved on heating ¾
Ans: HCOONa + NaOH ![]()
H 2 + Na2CO3.
Choice (C)
71. CH 3CH3 + HNO3 ¾
Ans: Choice (B)
72. When acetamide is hydrolysed by ¾
Ans: Choice (A)
73. Which will not go for diazotization?
Ans: Choice (B)
74. Secondary nitroalkanes can be ¾
Ans: 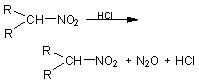
Choice(A)
75. ………. Stephen reduction to ¾
Ans: Alkyl cyanides on reduction with SnCl2 and HCl will give aldehyde.
Choice (A)
76. The continuous phase contains ¾
Ans: Choice (C)
77. The number of hydrogen atoms present in ¾
Ans: ![]() = 9.91 ´ 1023
= 9.91 ´ 1023
Choice (B)
78. Milk changes after digestion ¾
Ans: Choice (C)
79. ……… essential amino acids?
Ans: Choice (B)
80. ….. is a Ketohexose?
Ans: Choice (C)
VITEEE 2009 Sample Question Papers - Chemistry
VITEEE 2009 Sample Question Papers - Mathematics
VITEEE Information
VITEEE Past Trends, Cut-offs & Marks Distribution
Detailed Information - Vellore Institute Of Technology (VIT) This article is for all those people who have agood rank in aieee and also a good stream in vit. After seeing a no of aspirants in this forum looking for advice i try to spread a
source:-http://www.time4education.com